Hydrogen production method adopting electrochemistry to disintegrate HI in sulphur and iodine circulation hydrogen production and device
An electrochemical, sulfur-iodine technology, applied in the electrolysis process, electrolysis components, diaphragms, etc., can solve the problems of complex process, high equipment requirements, high energy consumption, etc., achieve mild conditions, low equipment requirements, simplify processes and equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
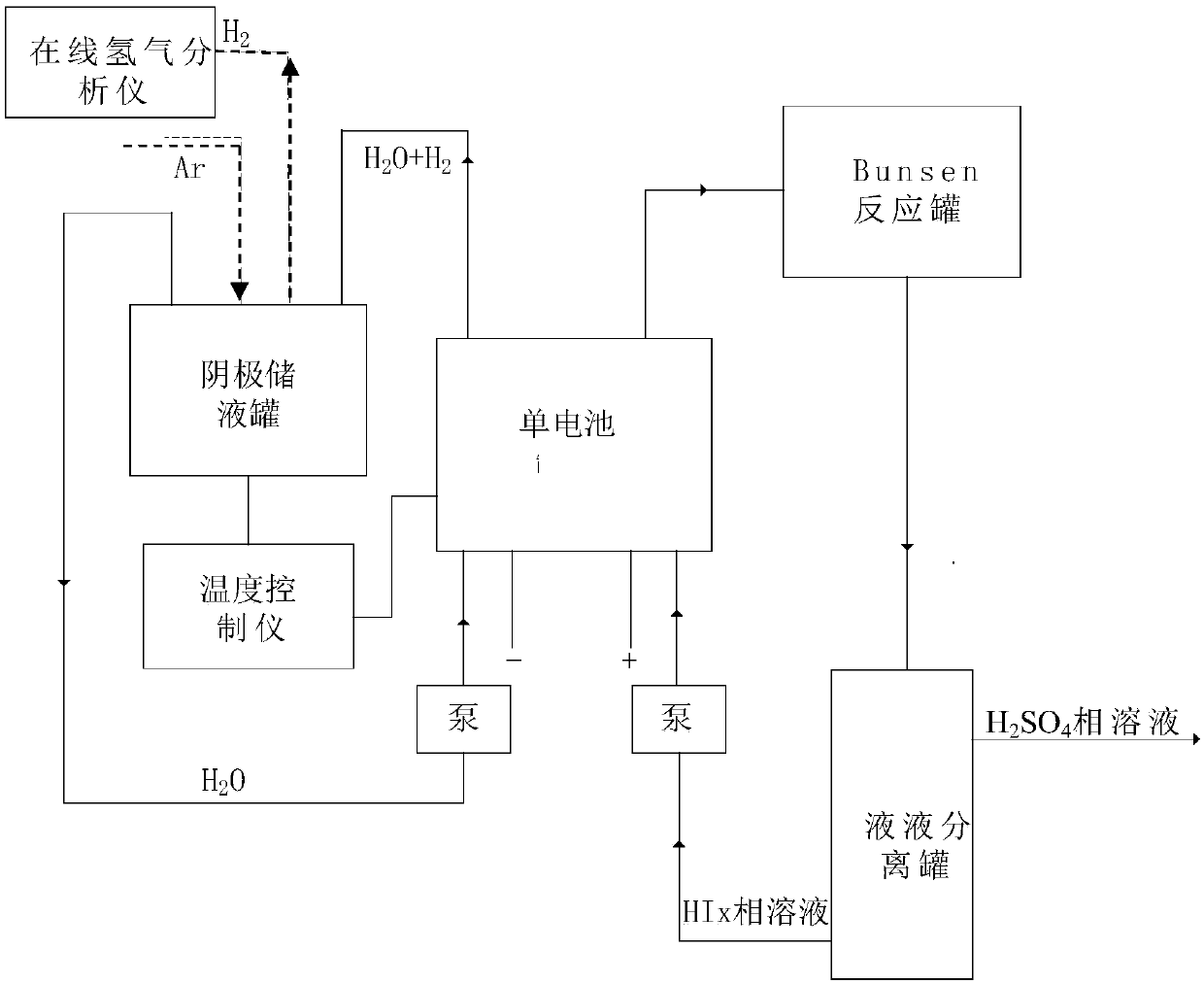
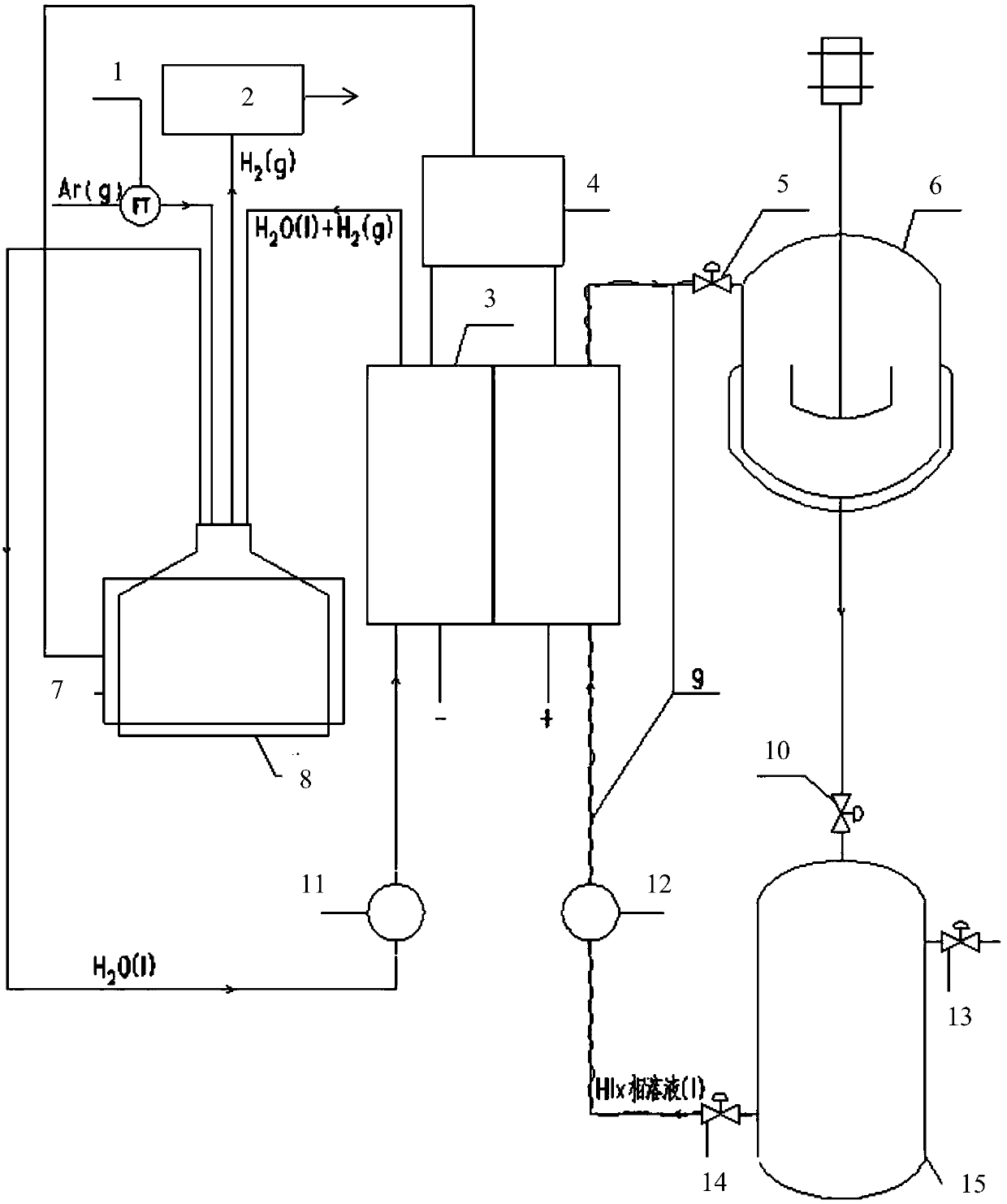
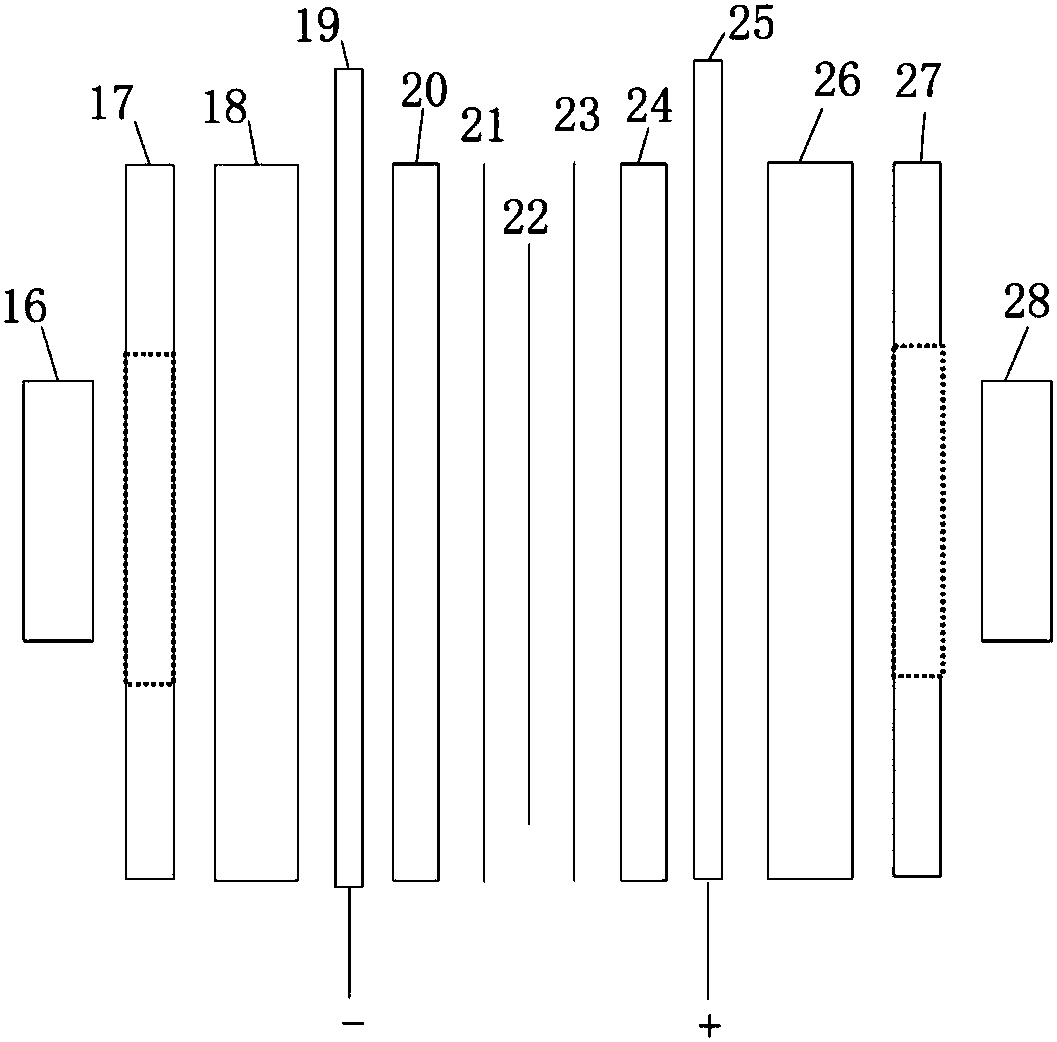
[0056] Two 10cm×10cm graphite electrodes with a thickness of 1cm were used as the cathode and anode electrodes respectively, and the N115 proton exchange membrane of 5.5cm×5.5cm (effective area 5cm×5cm) separated the cathode and yang graphite electrodes. Use a high temperature circulator and a water circulation system to control the temperature of the anode and cathode liquid storage tanks.
[0057] The operating temperature is respectively 30°C, 45°C, 60°C, and 75°C, and the molar concentration ratio is HI:I 2 :H 2 The HIx solution of O=1:1.2:6.17 was pumped into the anode of the single cell as the anolyte. The deionized water in the cathode storage tank is pumped into the cathode of the single cell. The temperature of the positive and negative electrodes of the electrolytic cell is controlled by the heating plate of the single cell and the temperature controller. Turn on the online hydrogen analyzer, and at the same time feed high-purity argon with a certain mass flow rat...
specific Embodiment 2
[0063] Two 10cm×10cm graphite electrodes with a thickness of 1cm were used as the cathode and anode electrodes respectively, and the N117 proton exchange membrane of 5.5cm×5.5cm (effective area 5cm×5cm) separated the cathode and yang graphite electrodes. When the operating temperature is 60°C, the molar concentration ratio is HI:I 2 :H 2 The HIx solution of O=1:0.6:6.17, 1:0.8:6.17, 1:1:6.17 and 1:1.2:6.17 is used as the anolyte to stabilize the current density at 100mA / cm 2 , the experimental results are as Figure 5 shown.
[0064] At 60°C, the current density is 100mA / cm 2 , the initial HI concentration is 9mol / kg H2O , with the initial iodine concentration from 5.4mol / kg H2O rise to 10.8mol / kg H2O , the current efficiency dropped from 92.36% to 91.30%, but it was basically in the range of 89-94%, and the energy consumption per mole of hydrogen production was from 648.58kJ / mol H2 rose to 787.85kJ / mol H2 . This shows that I 2 The higher the concentration is, the mo...
specific Embodiment 3
[0065] Referring to Example 1, a device for electrochemically decomposing HI to produce hydrogen was built, and the HIx phase solution was injected into the anode side of the single cell, and deionized water was injected into the cathode side of the single cell. The concentration of HI in the control solution is 6mol / kgH 2 O, the iodine element concentration is 7.2mol / kgH 2 O.
[0066] The decomposition reaction is carried out by electrification, and the current density in the single cell is controlled to be constant at 100mA / cm 2 , the operating reaction conditions are normal pressure, and the operating temperatures are 30°C, 45°C, 60°C, and 75°C respectively; the electrolysis voltage of the single cell is measured every two minutes; after the decomposition reaction, the HIx phase solution on the anode side is circulated back to the In the Bunsen reactor, the deionized water mixed with hydrogen on the cathode side is recycled back to the cathode liquid storage tank. Experi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com