Method for removing polar compounds from aromatic raw materials containing polar compounds
A technology of polar compounds and nitrogen compounds, used in carbon compound catalysts, hydrocarbons, hydrocarbons, etc., can solve the problems of limited capacity and low adsorption efficiency of adsorbents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
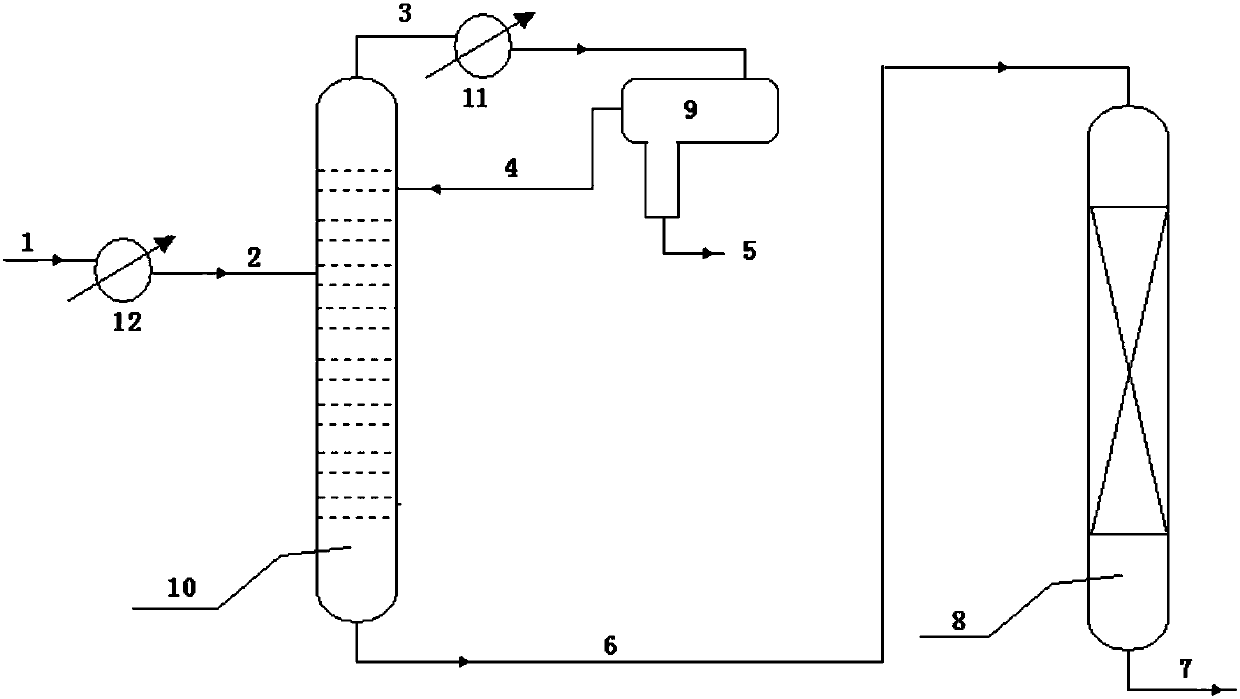
[0036] press figure 1 The process flow of the raw material benzene is 200ppm, the total nitrogen content is 3.0ppm, the total sulfur content is 0.7ppm, the rectification tower operating pressure is 0.01Pa, the top temperature is 80℃, and the bottom temperature is 82℃. The adsorbent bed is filled with 100g acid clay, the adsorbent bed temperature is 82℃, the pressure is 0.5MPa, and the benzene weight space velocity is 5.0h -1 . After continuous operation for 100 hours, the total nitrogen content at the outlet of the adsorbent bed was analyzed by a sulfur and nitrogen analyzer to be 0.05 ppm, and the total sulfur content was 0.06 ppm.
[0037] Using the purified benzene as the raw material, the alkylation of benzene and propylene is the target reaction. The reaction conditions are: fixed bed reactor, catalyst is acid Beta zeolite, reaction temperature 160℃, reaction pressure 3.0MPa, propylene weight space velocity 10.0h -1 , The molar ratio of benzene to propylene is 4.0, the cont...
Embodiment 2
[0040] press figure 1 The process flow of the raw material benzene contains 500ppm water, 3.0ppm total nitrogen, 1.6ppm total sulfur, rectification tower operating pressure 0.6MPa, tower top temperature about 140℃, and tower bottom temperature 160℃. The adsorbent bed is filled with 100g acid Beta zeolite, the adsorbent bed temperature is 160℃, the pressure is 2.0MPa, and the benzene weight space velocity is 3.0h -1 . After continuous operation for 100 hours, the total nitrogen content at the outlet of the adsorbent bed was analyzed by a sulfur-nitrogen analyzer to be 0.05 ppm, and the total sulfur content was 0.07 ppm.
[0041] Using the purified benzene as the raw material, the alkylation of benzene and propylene is the target reaction. The reaction conditions are: fixed bed reactor, catalyst is acid Beta zeolite, reaction temperature 160℃, reaction pressure 3.0MPa, propylene weight space velocity 10.0h -1 , The molar ratio of benzene to propylene is 4.0, the continuous reactio...
Embodiment 3
[0044] press figure 1 The process flow of raw material benzene contains 300ppm water, 10ppm total nitrogen, 1.6ppm total sulfur, rectification tower operating pressure 0.6MPa, tower top temperature about 140°C, and tower bottom temperature 160°C. The adsorbent bed is filled with 100g acid Beta zeolite, the adsorbent bed temperature is 160℃, the pressure is 2.0MPa, and the benzene weight space velocity is 3.0h -1 . After continuous operation for 100 hours, the total nitrogen content at the outlet of the adsorbent bed was analyzed by a sulfur-nitrogen analyzer to be 0.06 ppm, and the total sulfur content was 0.07 ppm.
[0045] Using the purified benzene as the raw material, the alkylation of benzene and propylene is the target reaction. The reaction conditions are: fixed bed reactor, the catalyst is acid MCM-22 zeolite, the reaction temperature is 160°C, the reaction pressure is 3.0MPa, and the weight of propylene Airspeed 10.0h -1 , The molar ratio of benzene to propylene is 4.0,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com