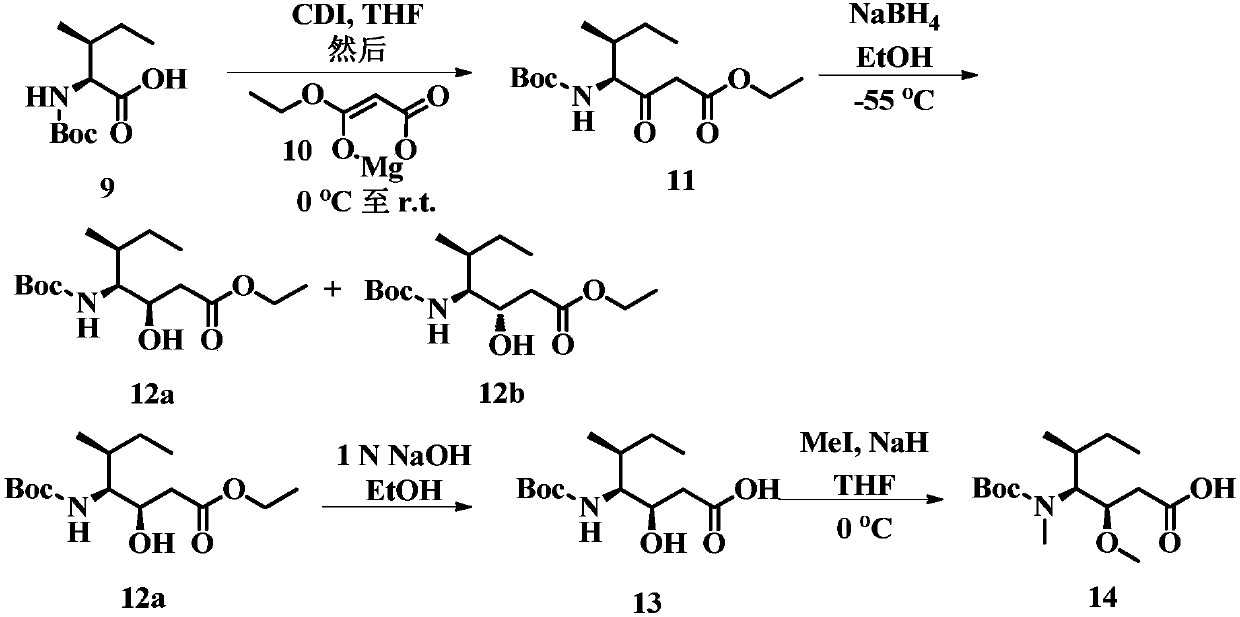
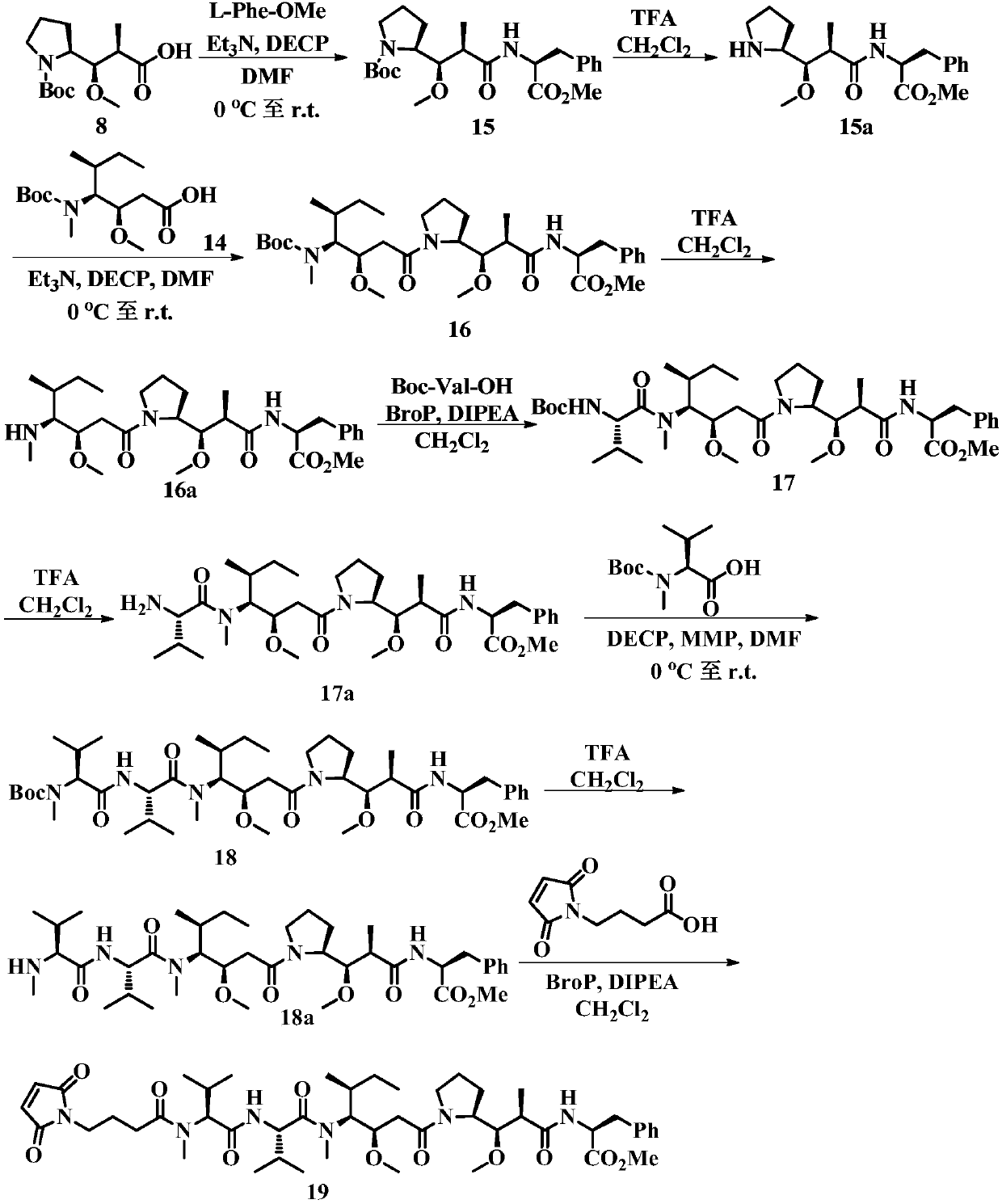
Auristatin analogues and their conjugates with cell-binding molecules
A technique of combining auristatin and molecules, which is applied in the field of derivatives of methyl auristatin F, which can solve the problems of reduced activity and unfavorable transport across cell membranes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
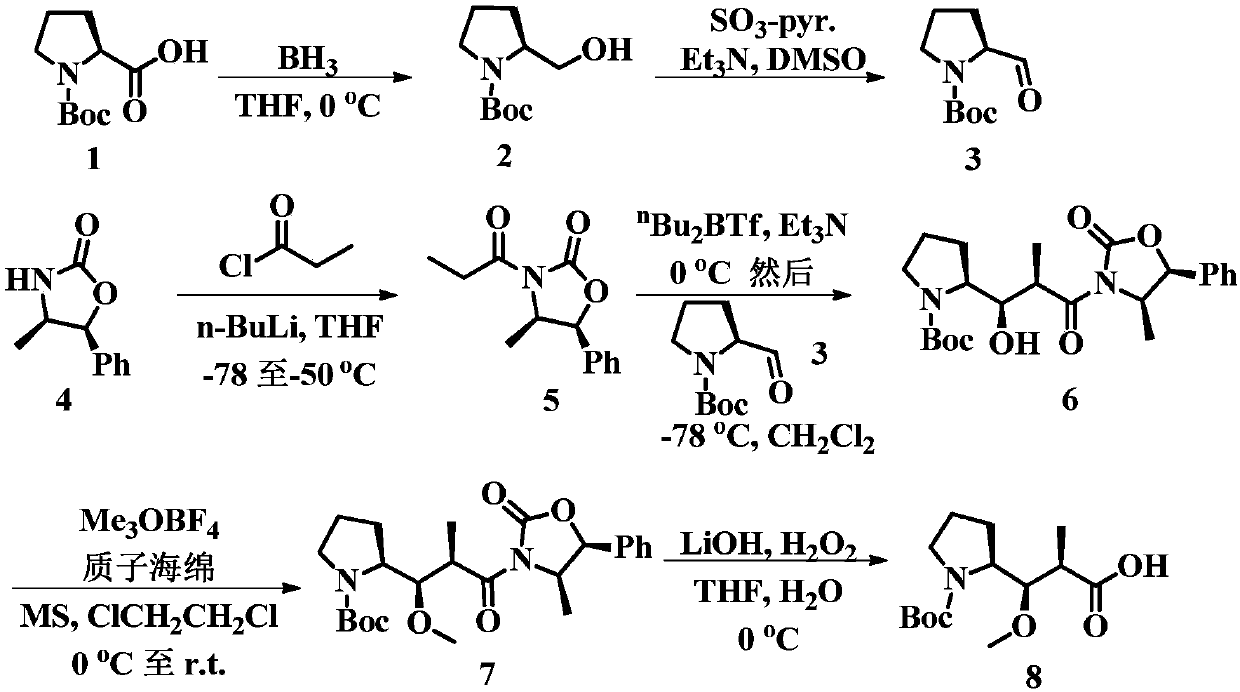
[0187] The preparation of embodiment 1 compound 2
[0188]
[0189] Dissolve Boc-L-proline (10.0 g, 46.4 mmol) in 50 mL of tetrahydrofuran and cool to 0° C., add BH 3 solution in tetrahydrofuran (1.0 M, 46.4 mL). The reaction was stirred at 0°C for 1.5 hours, poured into ice water and extracted with ethyl acetate. The organic phase was washed with brine (50 mL), dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain compound 2 (8.5 g, yield 91%) as a white solid. 1 H NMR (500MHz, CDCl 3 )δ3.94(dd,J=4.9,2.7Hz,2H),3.60(ddd,J=18.7,11.9,9.3Hz,2H),3.49–3.37(m,1H),3.34–3.23(m,1H) ,2.06–1.91(m,1H),1.89–1.69(m,2H),1.65–1.51(m,1H),1.49–1.40(m,9H).
Embodiment 2
[0190] The preparation of embodiment 2 compound 3
[0191]
[0192] To a solution of compound 2 (13.0 g, 64.6 mmol) in dimethylsulfoxide (90 mL) was added triethylamine (40 mL) and stirring was continued for 15 minutes. The mixture was cooled in an ice bath, and sulfur trioxide-pyridine complex (35.98 g, 226 mmol) was added portionwise over 40 minutes. The reaction was warmed to room temperature and stirred for 2.5 hours. After adding ice (250 g), the mixture was extracted with dichloromethane (150 mL×3). The organic phase was washed with 50% citric acid solution (150 mL), water (150 mL), saturated sodium bicarbonate solution (150 mL) and brine (150 mL), and dried over anhydrous sodium sulfate. Filtration and distillation under reduced pressure afforded aldehyde 3 (10.4 g, 81% yield) as a thick oil, which was used without further purification. 1 H NMR (500MHz, CDCl 3 )δ9.45(s,1H),4.04(s,1H),3.53(dd,J=14.4,8.0Hz,2H),2.00–1.82(m,4H),1.44(d,J=22.6Hz,9H ).
Embodiment 3
[0193] The preparation of embodiment 3 compound 5
[0194]
[0195] N 2 Under protection, a solution of n-butyllithium in n-hexane (21.6mL, 2.2M, 47.43mmol) was added dropwise to 4-methyl-5-phenyloxazolidin-2-one (8.0g, 45.17 mmol) in tetrahydrofuran (100 mL). The solution was stirred at -78°C for 1 hour, then propionyl chloride (4.4 mL, 50.59 mmol) was added slowly. The reaction mixture was naturally warmed to -50°C, stirred for 2 hours, and then quenched by adding saturated ammonium chloride solution (100 mL). The organic solvent was distilled off under reduced pressure, and the residual solution was extracted with ethyl acetate (3×100 mL). The organic layer was washed with saturated sodium bicarbonate solution (100 mL) and brine (100 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The concentrate was purified by column chromatography (20% ethyl acetate / n-hexane) to give compound 5 as a thick oil (10.5 g, 98% yield). 1 H NMR (500MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com