Role of citrullination in diagnosing diseases
A citrullination, disease technology, applied in the field of the role of citrullination in diagnosing diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: experimental method
[0092] Reagents and Materials
[0093] The following reagents were obtained: rabbit skeletal muscle PAD mix (PAD) (SignalChem); PAD2 (Sigma), heavy enzymatic myosin (HMM), tropomyosin (TM) (Sigma); F-actin (Cytoskeleton. Inc), cardiac troponin (TnI) (Abcam), anti-modified citrulline antibody (Millipore); sequencing grade Lys-C protease (WAKO), and protease inhibitor cocktail (Roche).
[0094] human heart tissue
[0095] Left ventricular tissue samples were obtained from Cris Dos Remedios, University of Sydney, Australia, after informed consent and approval by the local ethics committee. Samples were obtained from patients with HF (ischemic heart disease (ISHD) and idiopathic cardiomyopathy (IDCM), n=10 for each group) and non-heart failure donors during heart transplant surgery as previously described Heart (n=10) [Zhang P et al., Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the faili...
Embodiment 2
[0136] Identification of cardiac citrullinated proteins.
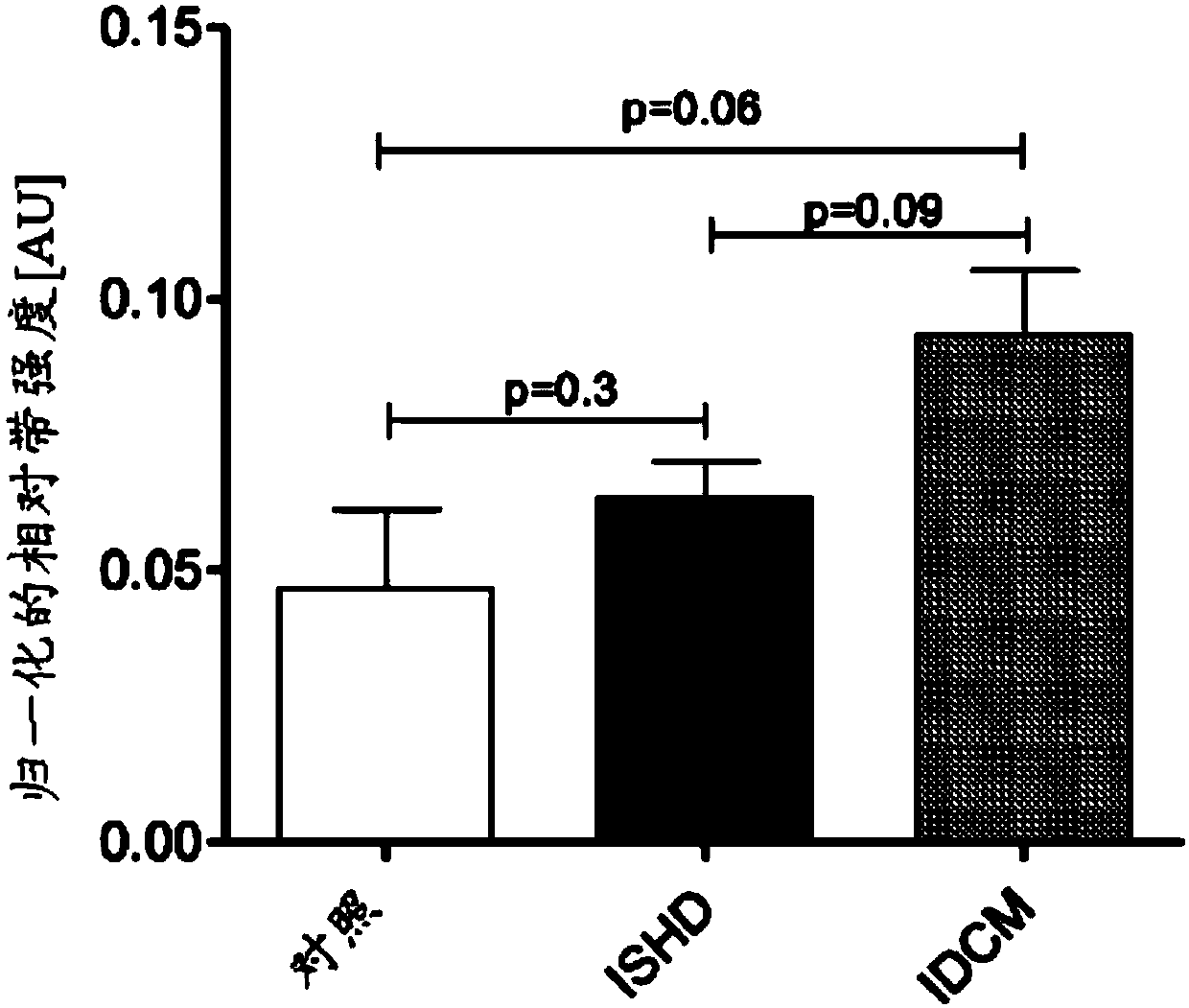
[0137] To identify citrullination targets in the heart, we assessed the citrullinome in three groups ISHD, IDCM and non-failing donor hearts (n=10 each) using SWATH-MS [34]. SWATH-MS allows for quantification. Compared to non-heart failure controls, 53 citrullination sites were altered with HF (p<0.05) and are listed in Table 1A.
[0138] Table 1A: List of citrullinated proteins (p=0.05) with citrullinated peptide sequences. "Dea" is citrullination or deamination. "CAM" is carbamide methylation. Citrullinated proteins are grouped by cellular components. UP identifier = universal protein (UP) resource protein ID; p_kw = p-value of Kruskal-Wallis test (statistic)
[0139]
[0140]
[0141]
[0142] Table 1B shows all citrullinated peptides present in this study. These peptides are proteins with diverse cellular functions, including regulators of transcription and chromatin structure, cytoskeleton and contr...
Embodiment 3
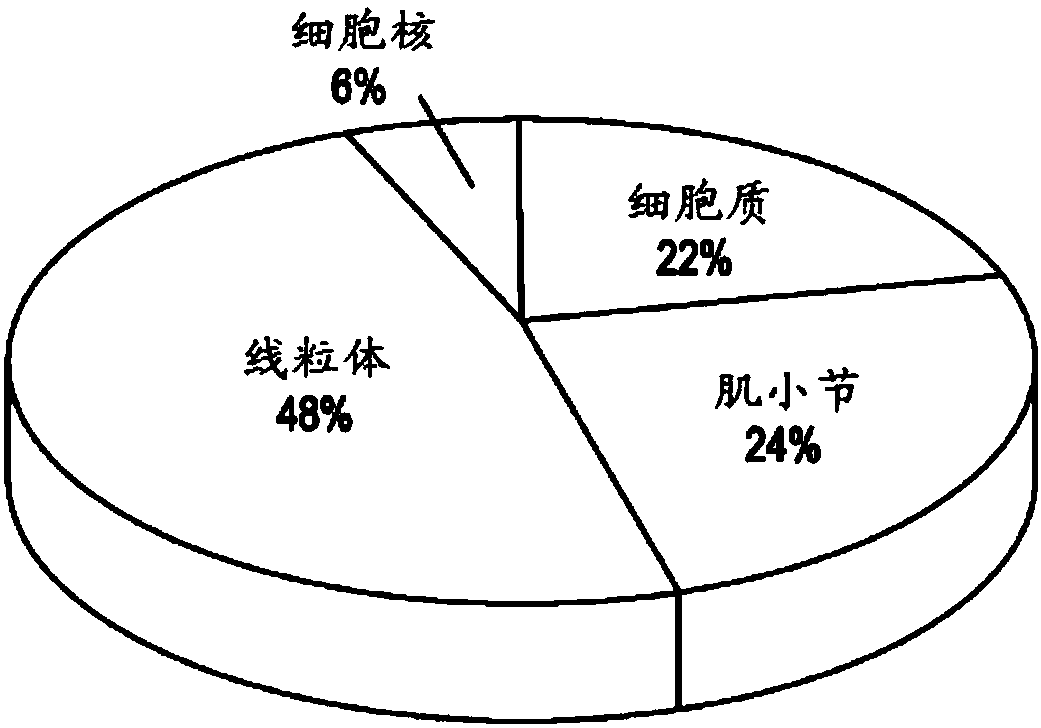
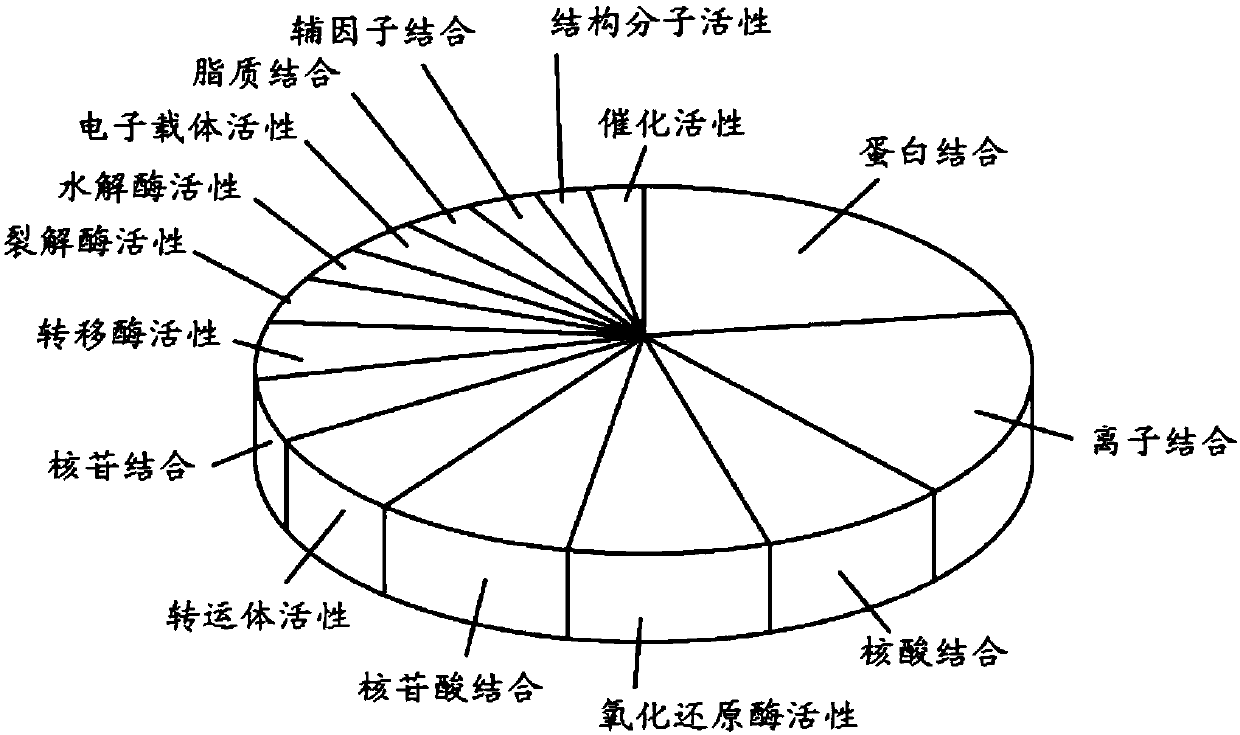
[0182] Our experimental results characterize citrullinated proteins in normal and HF myocardium. Analysis revealed that citrullination was enriched in the mitochondrial and sarcomere subproteomes. Protein citrullination has a broad cellular distribution (Fig. 1), but is highly enriched in the mitochondrial and sarcomere subproteomes. The close association between these two subproteomes is not unexpected due to the high energy requirements of sarcomeres. We speculate that citrullination, similar to phosphorylation and acetylation, may potentially regulate muscle contractile proteins in a coordinated manner. Interestingly, multiple enzymes involved in metabolic pathways / metabolism were upregulated in ischemia but downregulated in IDCM ( Figure 6 ). To understand the topology and functional annotation of citrullinated protein-protein interactions in the cardiac system, protein networks were constructed using the STRING database ( Figure 2B ). The entire protein network con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com