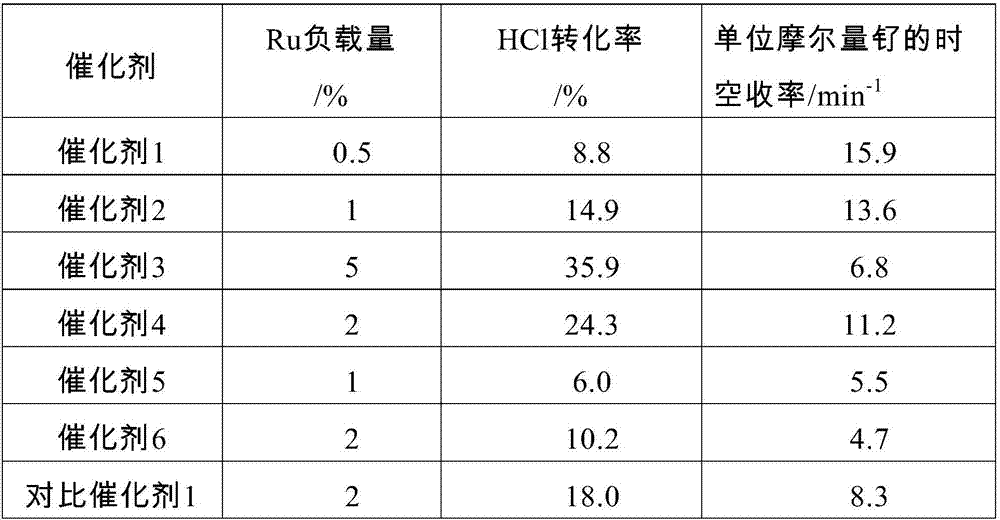
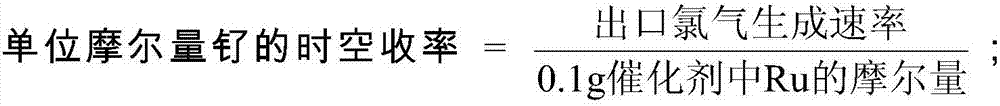Preparation method of hydrogen chloride oxidation catalyst
A technology of oxidation catalyst and hydrogen chloride, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, preparation with chloride, etc., which can solve the problem of low utilization efficiency of precious metal ruthenium, difficulty in industrial scale-up preparation, and water consumption Large and other problems, to achieve the effect of overcoming energy consumption and pollution, improving production yield, and reducing water consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The present embodiment provides a kind of preparation method of hydrogen chloride oxidation catalyst, and this method comprises the following steps:
[0029] Step 1, 0.14g commercially available RuCl 3 .nH 2 O (Ru mass content > 37%) was added to 50 g of distilled water to obtain a solution with a ruthenium mass content of 0.1%, named solution one;
[0030] Step 2, adding 20 g of commercially available 30% hydrogen peroxide into 20 g of distilled water to obtain a solution with a hydrogen peroxide content of 15%, named solution 2;
[0031] Step 3: According to the molar ratio of the ruthenium precursor, hydrogen peroxide, and titanium dioxide is 0.3:0.5:100, add the powdered titanium dioxide carrier (rutile type, Aladdin reagent) into the solution 1, and stir fully to obtain the solution 3; then add two drops of the solution Add solution three to obtain solution four;
[0032] Step 4: Warm up solution 4 to 80°C and keep it for 4 hours to obtain solution 5;
[0033] ...
Embodiment 2
[0037] The present embodiment provides a kind of preparation method of hydrogen chloride oxidation catalyst, and this method comprises the following steps:
[0038] Step 1, 0.68g of commercially available RuCl 3 .nH 2 O (Ru mass content > 37%) was added to 50 g of distilled water to obtain a solution with a ruthenium mass content of 0.5%, named solution one;
[0039] Step 2, add 20 g of commercially available 50% hydrogen peroxide to 20 g of distilled water to obtain a solution with a hydrogen peroxide content of 25%, which is named solution 2;
[0040] Step 3: According to the molar ratio of the ruthenium precursor, hydrogen peroxide, and titanium dioxide is 0.6:2:100, add the powdered titanium dioxide carrier (rutile type, Aladdin reagent) into the solution 1, and stir fully to obtain the solution 3; then add two drops of the solution Add solution three to obtain solution four;
[0041] Step 4: Warm up solution 4 to 90°C and keep it for 2 hours to obtain solution 5;
[0...
Embodiment 3
[0046] The present embodiment provides a kind of preparation method of hydrogen chloride oxidation catalyst, and this method comprises the following steps:
[0047] Step 1, 7.81g commercially available RuCl 3 .nH 2 O (Ru mass content > 37%) was added to 50 g of distilled water to obtain a solution with a ruthenium mass content of 5%, named solution one;
[0048] Step 2, a solution with a commercially available hydrogen peroxide content of 35%, named as solution 2;
[0049] Step 3: According to the molar ratio of the ruthenium precursor, hydrogen peroxide, and titanium dioxide is 3:10:100, add the powdered titanium dioxide carrier (rutile type, Aladdin reagent) into the solution 1, and stir fully to obtain the solution 3; then add two drops of the solution Add solution three to obtain solution four;
[0050] Step 4: Warm up solution 4 to 90°C and keep it for 2 hours to obtain solution 5;
[0051] Step 5, the pH of the solution 5 is adjusted to 6 or more with sodium metasili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com