Method for preparing 20-ene25-hydroxy rare ginsenosides and aglycones under catalysis of metal ions
A technology of ginseng rare saponins and metal ions, which is applied in the fields of organic chemistry and steroids, and can solve the problem of inability to prepare highly active 20-ene 25-hydroxyl rare saponins and ginsenosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
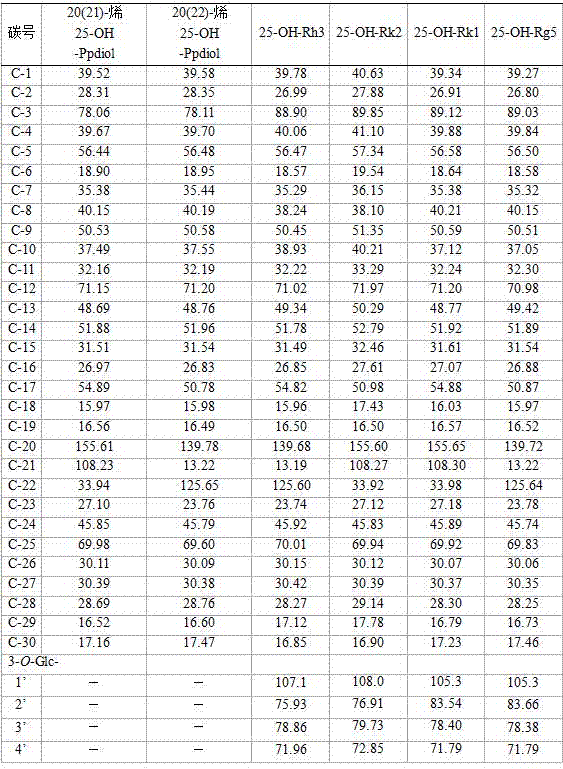
[0031] Example 1: Metal ions catalyze protopanaxadiol saponin Rb1 or Rb2 or Rb3 or Rc or Rd monomer or its monomer saponin mixture panaxadiol (PPD) saponin to prepare rare ginsenosides 25-OH-Rk1 and 25 -OH-Rg5 complex saponins.
[0032] Take 135 grams of FeCl 3 ·6H 2 O was dissolved in 1000 ml of absolute ethanol, and 11 grams of protopanaxadiol (PPD) saponins (including ginsenosides Rb1, Rb2, Rc, Rd) were added, stirred and dissolved, and stirred and reacted at 30-50°C for 6 hours; Add 1000 milliliters of water, and continue to stir and react at 30-50° C. for 24 hours. The reaction solution is subjected to repeated adsorption of saponin by 200 ml of macroporous adsorption resin, washed with 1200-1800 ml of water to remove salt ions and sugar and other impurities; then 400-700 ml of 50%-80% ethanol is used to elute saponin, The eluate was decolorized by 200 ml D-280 decolorization column, the ethanol eluate was collected, and about 6.5 grams of product was obtained after dr...
Embodiment 2
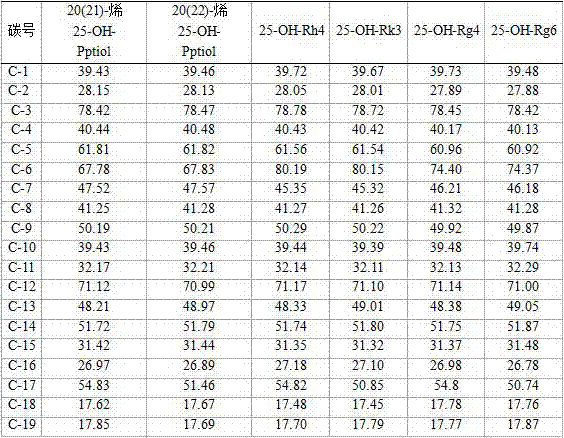
[0036] Example 2: Preparation of 25-OH-Rh3 and 25-OH-Rk2 complex saponins by metal ions catalyzing panaxadiol saponin F2 or Gyp17 or C-O or C-Mx1 or C-Mc1.
[0037] Take 150 grams of FeCl 2 Dissolve in 1000 milliliters of methanol, then add 10 grams of ginsengdiol saponin F2 and stir to dissolve, stir and react at 40-60°C for 7 hours; add 0.3-1.5 times the water of the reaction volume, and continue to react for 36 hours; after the reaction, Extract with 500 ml of water-saturated n-butanol for 3 to 4 times, combine the n-butanol layers, wash with a small amount of water for 2 to 4 times, concentrate under reduced pressure, and dry to obtain about 7.6 grams of 25-OH-Rh3 and 25-OH-Rk2 Mixture of isomers. Detected by high performance liquid chromatography (HPLC), the content of 25-OH-Rh3 and 25-OH-Rk2 compound saponins is more than 80%.
[0038] Use 10 grams of Gyp17 or C-O or C-Mx1 or Mc1 or their mixed saponins to replace F2 respectively, and under the same reaction conditions...
Embodiment 3
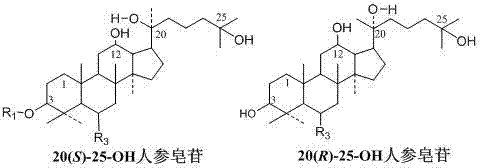
[0041] Example 3: Metal ions catalyze ginseng diol saponin C-K or Gyp75 or C-Y or C-Mx or C-Mc to prepare 20(21)-ene 25-OH-panaxadiol aglycon and 20(22)-ene 25 -OH-Panaxadiol aglycone.
[0042] Take 140 grams of FeCl 3 ·6H 2 O, completely dissolved in 1000 ml of methanol, add 10 g of ginsenoside C-K and stir to dissolve, at 40-60°C, stir and react for 8 hours; add 0.2-1.5 times the reaction volume of water, and continue the reaction for 24-48 hours. After the reaction is over, the reaction liquid is absorbed by a 250ml volume of macroporous adsorption resin to absorb saponin, washed with 2000ml (8 times the volume of the macroporous adsorption resin) of water to remove impurities such as salt ions and sugar, and then washed with 70%~95% ethanol The saponin was eluted, the ethanol eluate was collected, concentrated under reduced pressure, and dried to obtain 7.2 g of the product. The content of 20(21)-ene 25-OH-panaxadiol aglycon and 20(22)-ene 25-OH-panaxadiol aglycon was o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com