A kind of preparation method and application of high-purity mannoglycan
A mannanase, high-purity technology, applied in the field of preparation of high-purity mannan, can solve the problems of increased solubility, long irradiation time, and reduced viscosity of KGM hydrosol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
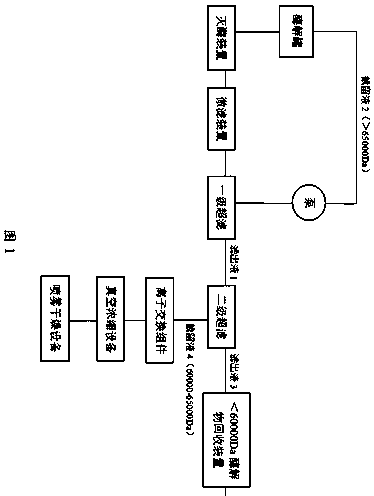
[0034] Example 1: as figure 1 Shown is a preparation method of mannan, comprising the following steps:
[0035] (1) Add 200kg of production water to the enzymolysis tank, control the temperature in the enzymolysis tank to 30-50°C, and put neutral β-mannanase into the enzymolysis tank (the amount of enzyme input is based on the amount of enzyme and konjac extract Powder mass ratio is 1:10-30), weigh 10kg of konjac fine powder, put it evenly into the enzymolysis tank with rapid and automatic detection material viscosity device, control the pH of the material in the enzymolysis tank to 6.0-7.5, with the enzyme As the hydrolysis time advances, the viscosity of the enzymolysis solution drops rapidly. When the viscosity real-time detection device installed on the enzymolysis tank detects that the viscosity of the enzymolysis solution reaches 1100-1200mpa.s, the enzyme is immediately deactivated, and the deactivation time is 20-30min. cool down;
[0036] (2) Microfiltration to remo...
effect experiment Embodiment example 1
[0062] Effect Experiment Implementation Case 1: Mannoside Glycan Used in Anti-tumor Experiment
[0063] An application of the above-mentioned produced mannosidose in anti-tumor. Mannosidose is made into corresponding products or applied in anti-tumor products, which has obvious inhibitory effect on tumors. The ratio to body weight is 100-300mg / kg.
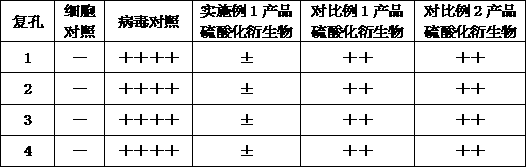
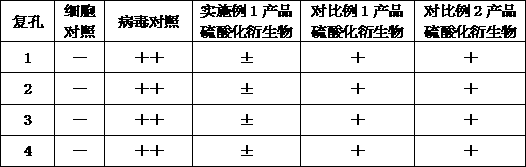
[0064] Anti-tumor test plan: select mice inoculated with tumors for 10 days, aseptically extract ascites from mice, dilute with 0.9% sodium chloride injection 3 times, adjust the number of tumor cells to 1×108 / ml, and give each mouse axillary Inoculate 0.2ml subcutaneously. Randomly grouped into groups, and started administration 24 hours after inoculation. The control group received the same amount of normal saline, once a day; the 5-fluorouracil group was 10 mg / kg, subcutaneously injected, once a week; the product of Example 1 and the product of Comparative Example 1 1. Each 200 mg / kg product of Comparative Example 2 was admini...
effect experiment Embodiment example 2
[0069] Effect experiment implementation case 2: Antiviral test of mannosidin sulfated derivatives
[0070] An application of the above-mentioned produced mannosidose in anti-virus, wherein the mannosidose is subjected to sulfate modification treatment to make corresponding products or apply it to anti-virus products, so that the sulfated mannosidose When the concentration is above 200μg / ml, it has a good broad-spectrum antiviral effect.
[0071] Sulfated polysaccharides refer to natural and semi-synthetic acidic polysaccharides containing sulfuric acid groups, which are polyanionic compounds. Since the discovery of sulfated dextran in 1987 that it has the activity of inhibiting AIDS virus HIV, the antiviral aspects of this type of polysaccharides The research is very active, and it may become another class of HIV-1 inhibitors following viral reverse transcriptase activity inhibitors and protease inhibitors. Sulfated mannan is modified by sulfation of mannan by chlorosulfonic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com