Preparation method of high-purity mannan and application of high-purity mannan
A mannanase and high-purity technology, which is applied in the field of preparation of high-purity mannan, can solve the problems of small test treatment volume, long irradiation time, and low purity, and achieve important economic and production values and operational processes. Simple, optimized preparation process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
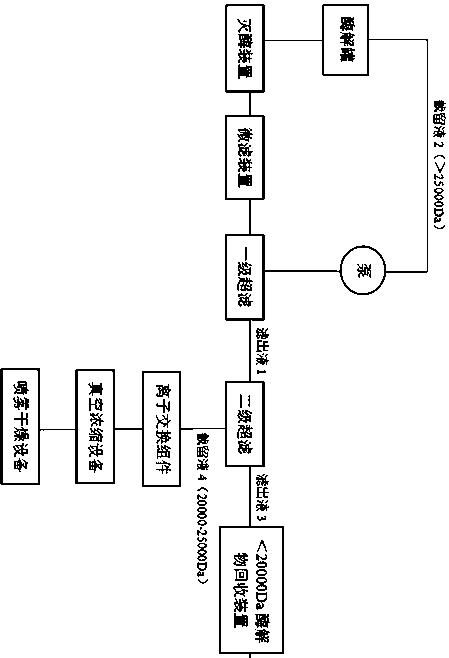
[0035] Example 1: as figure 1 Shown is a preparation method of mannan, comprising the following steps:
[0036] (1) Add 200kg of production water to the enzymolysis tank, control the temperature inside the enzymolysis tank to be 30-50°C, and put neutral β-mannanase into the enzymolysis tank (the amount of enzyme input is based on the amount of enzyme and konjac extract Powder mass ratio is 1:10-30), weigh 10kg of konjac fine powder, put it evenly into the enzymolysis tank with rapid and automatic detection material viscosity device, control the pH of the material in the enzymolysis tank to 6.0-7.5, with the enzyme As the hydrolysis time advances, the viscosity of the enzymolysis solution drops rapidly. When the viscosity real-time detection device installed on the enzymolysis tank detects that the viscosity of the enzymolysis solution reaches 700-800mpa.s, the enzyme is immediately deactivated, and the deactivation time is 20-30min. cool down;
[0037] (2) Microfiltration to...
effect experiment Embodiment example 1
[0063] Effect experiment implementation case 1: Mannosidan used in hypoglycemic experiment
[0064] Experimental method: Select 60 Kunming adult mice (half male and half male), with an average body weight of (25±2.5) g, randomly select 10 mice as the normal control group according to the principle of half male and half male, and feed the remaining 50 mice with high-sugar feed for 1 -After 2 months, diabetes was induced with small doses of streptozotocin, and those whose fasting blood glucose value was greater than 20mmol / L were included in the experiment, and then the mice were randomly divided into a type II diabetes model group, a metformin administration group, and the product of Example 1 The administration group, the product administration group of Comparative Example 1 and the product administration group of Comparative Example 2; the normal control group and the type II diabetes model group were given the same amount of distilled water. The experimental period was 4 wee...
effect experiment Embodiment example 2
[0071] Effect Experiment Implementation Case 2: Anticoagulant Test of Mannan Sulfated Derivatives
[0072] experimental method:
[0073] The products of Example 1, Comparative Example 1, and Comparative Example 2 were subjected to sulfate modification treatment to obtain mannan sulfated derivatives with good water solubility.
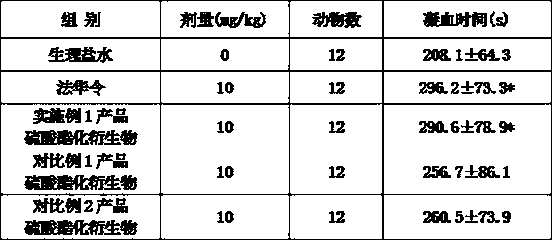
[0074] Select 60 Kunming adult mice (half male and half male), with an average body weight of (25±2.5) g, randomly divide them into 5 groups, 12 mice in each group. Gavage according to the capacity of 25ml / kg, respectively give normal saline, warfarin, the sulfated derivative of the product of embodiment 1, the sulfated derivative of the product of comparative example 1, and the sulfated derivative of the product of comparative example 2, each 10mg / kg , continuous gavage for 30d. One hour after administration on the 30th day, blood was collected from the venous plexus behind the inner canthus of the mouse eyes, and the coagulation time of the mice in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com