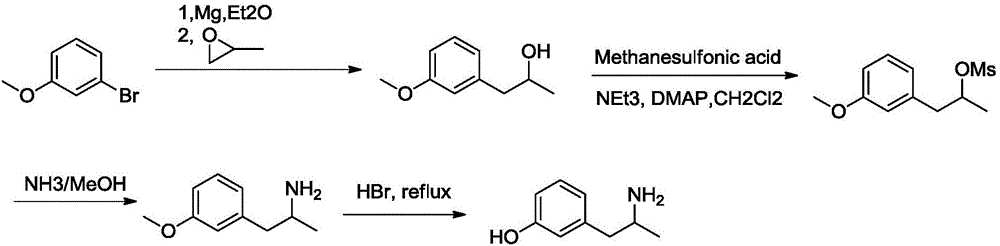
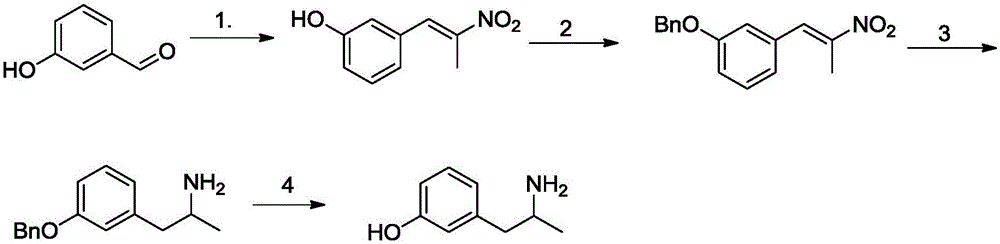
Method for preparing 3-(2-aminopropyl)phenol
A technology of aminopropyl and phenol, which is applied in the field of preparation of 3-phenol, can solve the problems of complex post-processing operation, unfavorable industrial production, and poor production safety, so as to simplify the reaction process and post-processing process, reduce production risks, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The first step: the synthesis of 1-(3-methoxyphenyl)isopropanol.
[0034] A solution of m-methoxybromobenzene (481mmol, 90g) in tetrahydrofuran (400ml) was slowly added dropwise to a solution of 1,2-dibromoethane (4ml) and magnesium chips (481mmol, 11.7g) in ether (400ml) , the reaction mixture was stirred and refluxed until the magnesium chips were dissolved. Propylene oxide (433mmol, 25.15g) was then added to the reaction mixture at 30°C, and the reaction was allowed to react at this temperature for 20 minutes, then the temperature was raised to room temperature and stirred for 1 hour. The reaction was quenched with aqueous ammonium chloride solution (600ml), and extracted with diethyl ether (2000ml), the organic layer was washed with water and brine successively, dried over sodium sulfate, spin-dried and cross-eluted column (eluent: petroleum ether / ethyl acetate= 4 / 1) 1-(3-methoxyphenyl)isopropanol (64.7g, yield: 80.89%) was obtained as an oily substance.
[0035] ...
Embodiment 2
[0049] The first step: the synthesis of 1-(3-methoxyphenyl)isopropanol.
[0050] A solution of m-methoxybromobenzene (481mmol, 90g) in ether (400ml) was slowly added dropwise to a solution of 1,2-dibromoethane (4ml) and magnesium chips (481mmol, 11.7g) in ether (400ml) , the reaction mixture was stirred and refluxed until the magnesium chips were dissolved. Then propylene oxide (457mmol, 26.5g) was added to the reaction mixture at 30°C, the reaction was allowed to react at this temperature for 20 minutes, then the temperature was raised to room temperature and stirred for 1 hour. The reaction was quenched with aqueous ammonium chloride solution (600ml), and extracted with diethyl ether (2000ml), the organic layer was washed with water and brine successively, dried over sodium sulfate, spin-dried and cross-eluted column (eluent: petroleum ether / ethyl acetate= 4 / 1) 1-(3-methoxyphenyl)isopropanol (65.6 g, yield: 82%) was obtained as an oily substance.
[0051] Through nuclear m...
Embodiment 3
[0063] The first step: the synthesis of 1-(3-methoxyphenyl)isopropanol.
[0064] A solution of m-methoxybromobenzene (481mmol, 90g) in 1,2-dibromoethane (400ml) was slowly added dropwise to 1,2-dibromoethane (4ml) and magnesium chips (481mmol, 11.7g) In a solution of diethyl ether (400ml), the reaction mixture was stirred at reflux until the magnesium chips were completely dissolved. Propylene oxide (409 mmol, 23.8 g) was then added to the reaction mixture at 30° C., the reaction was allowed to react at this temperature for 20 minutes, then the temperature was raised to room temperature and stirred for 1 hour. The reaction was quenched with aqueous ammonium chloride solution (600ml), and extracted with diethyl ether (2000ml), the organic layer was washed with water and brine successively, dried over sodium sulfate, spin-dried and cross-eluted column (eluent: petroleum ether / ethyl acetate= 4 / 1) 1-(3-methoxyphenyl)isopropanol (64.1 g, yield: 80.1%) was obtained as an oily subst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com