A kind of synthetic method and application of morphine derivative
A synthetic method and technology of morphine, applied in the field of analgesics, can solve the problems that it cannot be used as a standard or reference substance, and the purity of M3G cannot be obtained, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
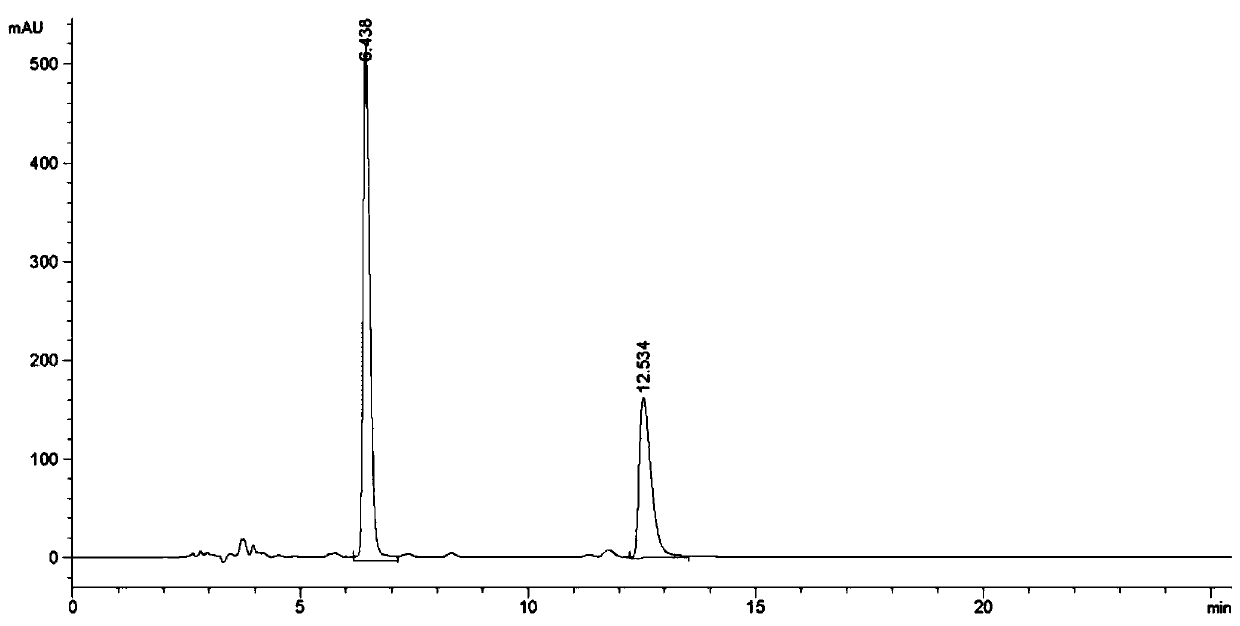
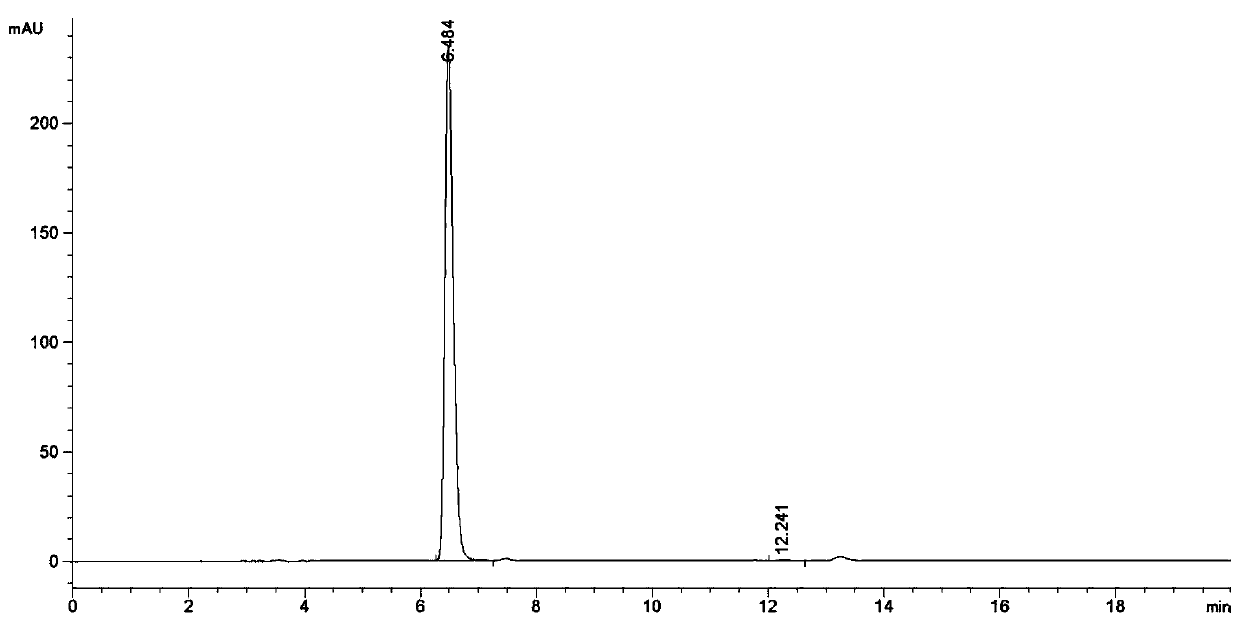
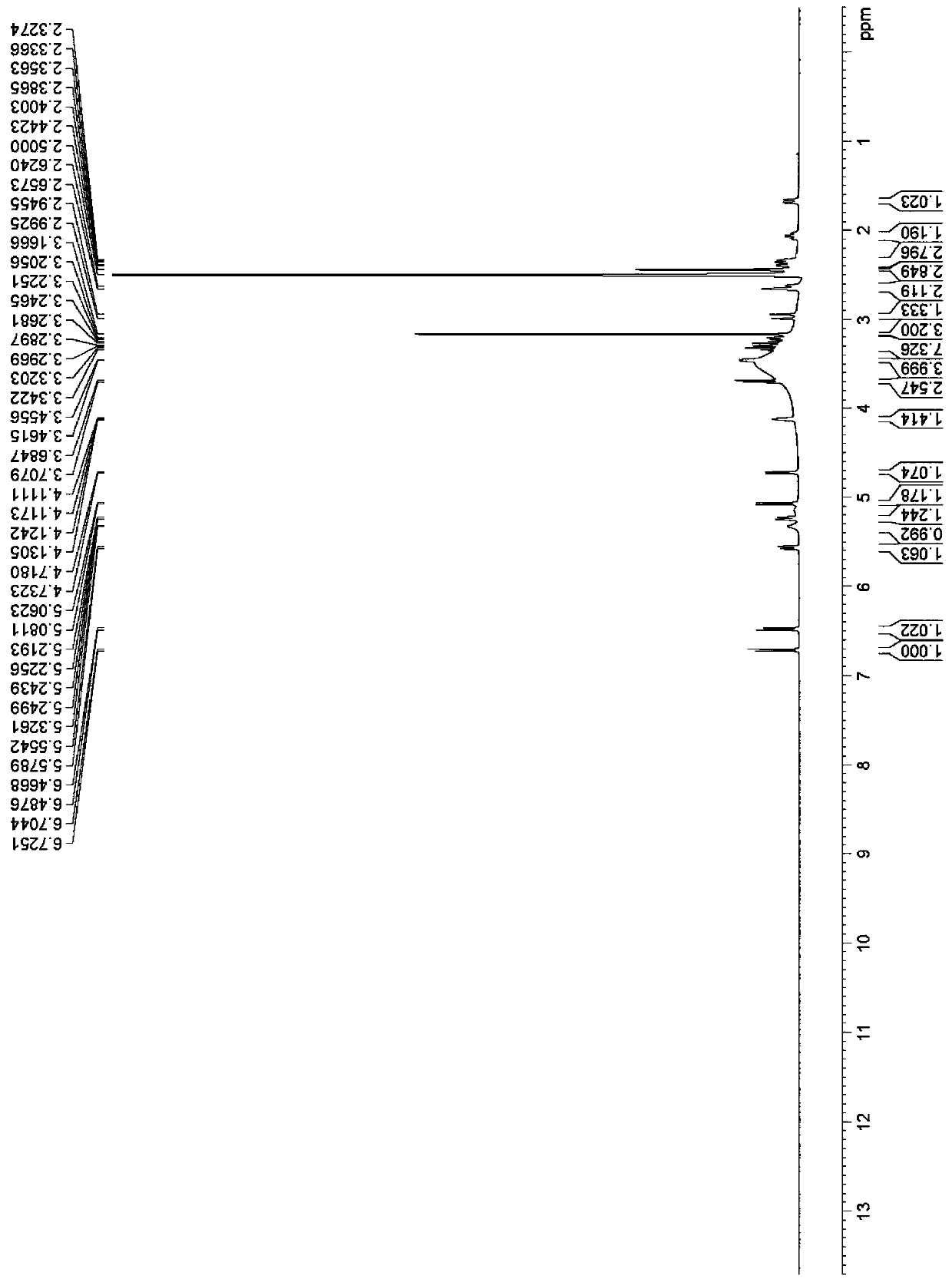
Image
Examples
Embodiment 1
[0062] (1) compound shown in formula II (R 1 for the synthesis of acetyl)
[0063] Take 10g of morphine and add it to acetonitrile, add 7.1g of triethylamine, lower to 0°C, add dropwise 7.5g of acetic anhydride, keep the temperature below 10°C, after dropping, react at 20-30°C for 5 hours, pour the reaction solution into 100ml Chloromethane, neutralized with saturated sodium bicarbonate solution, washed with 50ml of purified water and saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
[0064] (2) compound shown in formula III (R 1 is acetyl) and formula IV compound (R 5 for the synthesis of trifluoroacetyl)
[0065] Dissolve the product obtained in the first step with 150ml of absolute ethanol, add 2.4g of hydroxylamine hydrochloride, react at 20-30°C for 12 hours, concentrate under reduced pressure, add 50ml of water and 120ml of dichloromethane to the concentrate, extract and separate, and dichloromethane...
Embodiment 2
[0073] (1) compound shown in formula II (R 1 for the synthesis of benzoyl)
[0074] Add 10g of morphine into tetrahydrofuran, add 2.8g of sodium hydride, lower to 0°C, add dropwise 10.2g of benzoyl chloride, keep the temperature below 10°C, after the drop is complete, react at 20-30°C for 1 hour, pour the reaction solution into 100ml of dihydrofuran Chloromethane, neutralized with saturated sodium bicarbonate solution, washed with 50ml of purified water and saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
[0075] (2) compound shown in formula III (R 1 Be benzoyl) and formula IV compound (R 5 for the synthesis of trifluoromethanesulfonyl)
[0076] Dissolve the product obtained in the first step with 120ml of anhydrous methanol, add 3.8g of phenylhydrazine, react at 30-40°C for 10 hours, concentrate under reduced pressure, add 50ml of water and 120ml of dichloromethane to the concentrate, extract and separa...
Embodiment 3
[0084] (1) compound shown in formula II (R 1 for the synthesis of 3,5-dinitrobenzoyl)
[0085] Take 10g of morphine and add it to acetone, add 5.5g of pyridine, lower to 0°C, add dropwise 16.2g of 3,5-dinitrobenzoyl chloride, keep the temperature below 10°C, after dropping, react at 10-20°C for 5 hours, and The reaction solution was poured into 100ml of dichloromethane, neutralized with saturated sodium bicarbonate solution, washed with 50ml of purified water and saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
[0086] (2) compound shown in formula III (R 1 is 3,5-dinitrobenzoyl) and formula VI compound (R 5 for the synthesis of trifluoroacetyl)
[0087] Dissolve the product obtained in the first step with 150ml of isopropanol, add 4.8g of anhydrous potassium carbonate, react at 50-60°C for 12 hours, concentrate under reduced pressure, add 50ml of water and 120ml of dichloromethane to the concentrate, ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com