A kind of protein for enhancing immune effect of pig vaccine and its application
An immune effect and protein technology, applied in the field of genetic engineering, can solve the problem of less application, and achieve the effects of simple preparation operation, good specificity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Obtaining nucleic acid molecules of proteins for enhancing the immune effect of porcine vaccines, the method is as follows:
[0040] Extraction of porcine total RNA: Extract porcine total RNA from tissues such as porcine spleen or lymph nodes according to the instructions of Promega’s RNA extraction kit.
[0041] Reverse transcription and PCR of IL-33 target gene: reverse transcription was carried out according to the RT-PCR kit of Promega Company, and the cDNA of IL-33-mRNA was obtained. Referring to the porcine IL-33 gene sequence (GeneBank number: AB292180), the following primers were designed:
[0042] IL-33-F120: cccatatgagtatcaaagaacattctgct
[0043] IL-33-R276: ccctcgagcattaagtttgagagcttaaatg
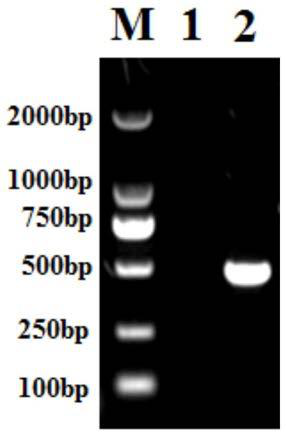
[0044] Using cDNA as a template, use the designed primers for PCR amplification:
[0045] The PCR reaction conditions were as follows: pre-denaturation at 95°C for 5 minutes, 1 cycle; denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72...
Embodiment 2
[0050] Construct Escherichia coli expression vector IL-33 / pET28a(+), the method is as follows:
[0051] (1) After the PCR amplification product obtained in Example 1 was double-digested with restriction endonucleases NcoI and XhoI, the digested product was recovered.
[0052] (2) Digest the vector pET28a(+) with restriction endonucleases NcoI and XhoI to recover the vector backbone.
[0053] (3) Ligate the digested product of step (1) with the vector backbone of step (2) with T4 DNA ligase to obtain a ligation product.
[0054] (4) Transform the ligation product of step (3) into Escherichia coli Rosetta (DE3) competent cells, pick out single clones for PCR identification, and carry out sequencing identification for positive clones. The sequencing results show that the recombinant plasmid IL-33 / pET28a was obtained .
[0055] Description of the structure of the recombinant plasmid IL-33 / pET28a: the DNA shown in sequence 2 of the sequence table is inserted between the NcoI and Xh...
Embodiment 3
[0057] Prepare recombinant cells containing the carrier IL-33 / pET28a(+), the method is as follows:
[0058] Escherichia coli Rosetta (DE3) containing pET28a-IL-33 was named Rosetta-IL-33 / pET28a to obtain recombinant cells.
[0059] The obtained recombinant cells Rosetta-IL-33 / pET28a and the vector Rosetta / pET28a obtained in Example 2 were respectively inoculated in LB medium containing 100 mg / mL kanamycin, and cultured with shaking at 37°C and 150 rpm until OD600=1 , and then add IPTG to a final concentration of 0.3mM, and induce at 37°C for 4h.
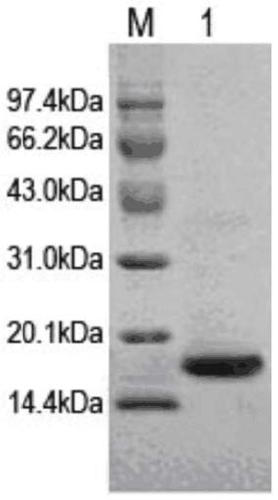
[0060] Centrifuge the pre- and post-induction bacterial solutions of Rosetta-IL-33 / pET28a and Rosetta / pET28a at 12,000rpm for 10min, collect the bacterial cells, resuspend them in PBS, and ultrasonically break the bacteria, centrifuge at 12,000rpm for 15min, and take the supernatant and precipitate separately Perform SDS-PAGE electrophoresis.
[0061] According to the results of SDS-PAGE electrophoresis, the detection result of Ros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com