Polyanthracene compound, and preparation method and application thereof
A compound and polymer technology, applied in polyanthracene and its synthesis method, and in the application field of polymer light-emitting diodes, can solve the problems of troublesome processing, deep color, wide spectrum, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of 9,10-dibromoanthracene, the synthetic route is as follows:
[0036]
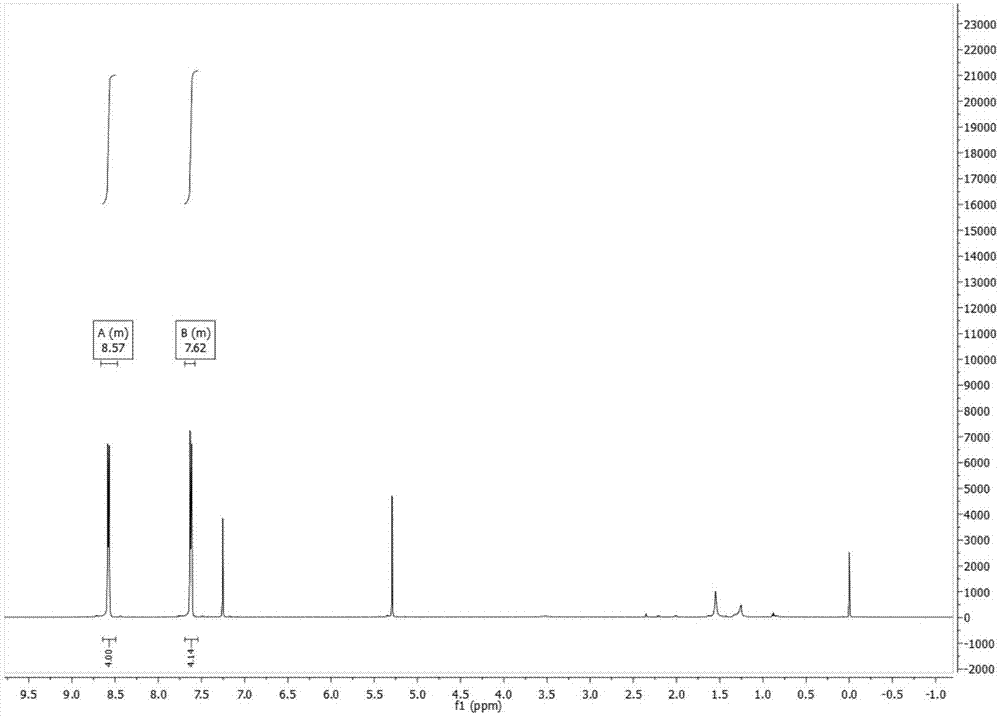
[0037] Add anthracene (10g, 56.1mmol) and 200ml chloroform in a 1000ml single-necked flask, stir and dissolve at room temperature, add liquid bromine (18g, 112.6mmol) and 100ml chloroform in a 250ml constant pressure funnel, and keep the reaction device away from light. And the ventilation tube on the constant pressure funnel was piped into the sodium hydroxide solution, and stirred at room temperature for 4 hours. Add sodium hydroxide solution to quench, after layering, wash the organic layer with brine, add anhydrous magnesium sulfate to dry, spin off the solvent after Buchner funnel filtration, and dry it with a vacuum pump to obtain a yellow solid, which is recrystallized with toluene to obtain 16.7 g of yellow needles, yield 88.6%. 1 H NMR (600MHz, CDCl 3 )δ8.67–8.47(m,4H),7.69–7.58(m,4H).
[0038] Add 9,10-dibromoanthracene (0.25g, 0.744mmol) and activated magnesium (0.0...
Embodiment 2
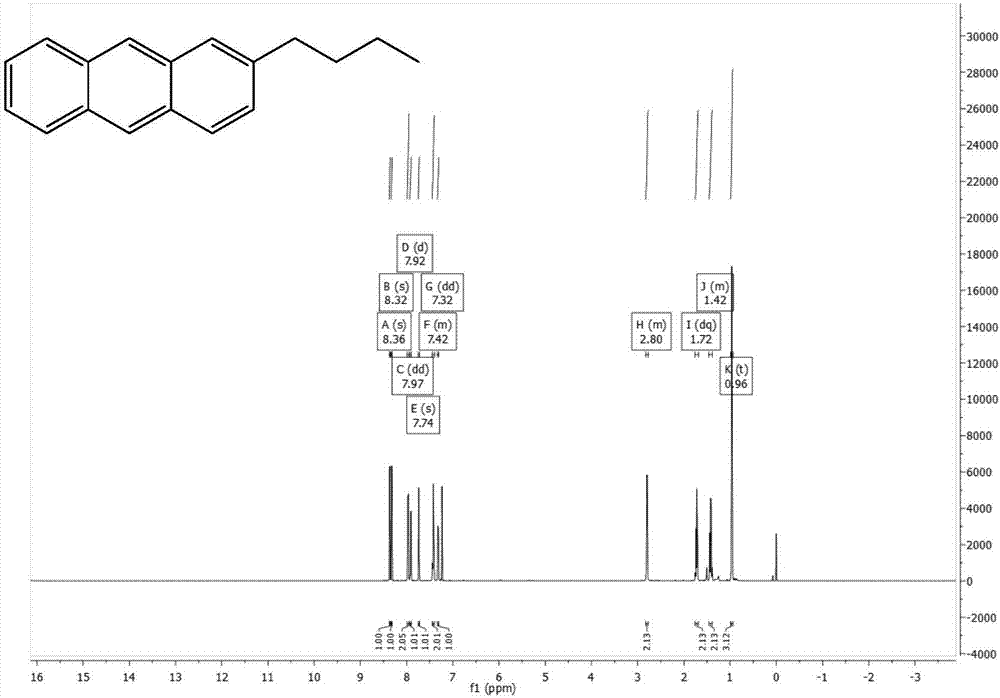
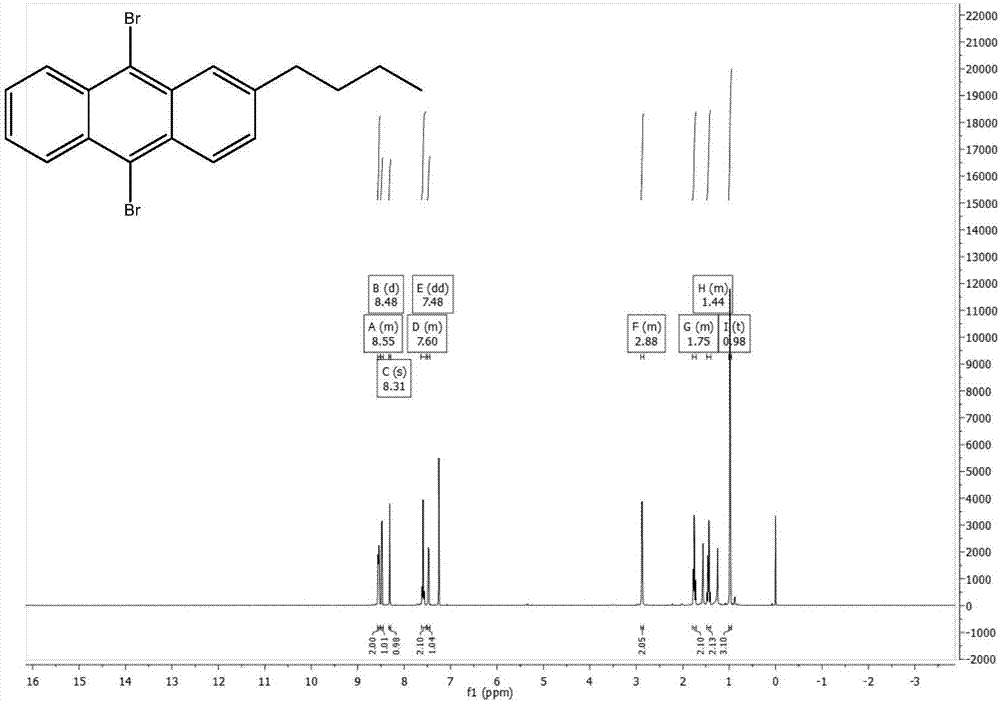
[0040] The preparation of 2-n-butyl-9,10-dibromoanthracene, the synthetic route is as follows:
[0041]
[0042] Add phthalic anhydride (11.11g, 75mmol), anhydrous aluminum chloride (24g, 180mmol), and 50ml of chloroform into a three-necked flask, and add n-butylbenzene (10.07g, 75mmol) and 50ml of chloroform into a constant pressure funnel , drop slowly, react at 50°C for 1 hour after the drop is completed, then rise to cool, pour into a mixed solution of 48ml concentrated hydrochloric acid and 96g ice to quench, add chloroform to extract three times, add anhydrous magnesium sulfate to dry, and use Brinell After filtering through the funnel, the solvent was evaporated by rotary evaporation, and dried by vacuum pump to obtain 21.09 g of brown solid, with a crude yield of 99.6%.
[0043] Add 2-(3-n-butylbenzoyl)benzoic acid (21.09g, 74.7mmol) and 126ml of concentrated sulfuric acid into a single-necked flask, react at 125°C for one hour, pour into ice to quench, extract with...
Embodiment 3
[0048] The preparation of 2-tert-butyl-9,10-dibromoanthracene, the synthetic route is as follows:
[0049]
[0050] Weigh phthalic anhydride (11.11g, 75mmol), anhydrous aluminum chloride (24g, 180mmol), and 50ml of chloroform in a single-necked bottle, and add tert-butylbenzene (10.07g, 75mmol) and chloroform into the constant pressure funnel Add 50ml slowly, react at 50°C for 1 hour after the drop, rise to cool, pour into a mixed solution of 48ml concentrated hydrochloric acid and 96g ice to quench, add chloroform to extract three times, add anhydrous magnesium sulfate to dry, Brinell After filtering through the funnel, the solvent was evaporated and sucked to dryness to obtain 21.68 g of a brown solid, and the crude yield was greater than 100%.
[0051] Add 2-(3-tert-butylbenzoyl)benzoic acid (4.35g, 15.4mmol) and 25ml concentrated sulfuric acid into a single-necked bottle, react at 125°C for one hour, pour into ice to quench, extract with toluene, add anhydrous Dry over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com