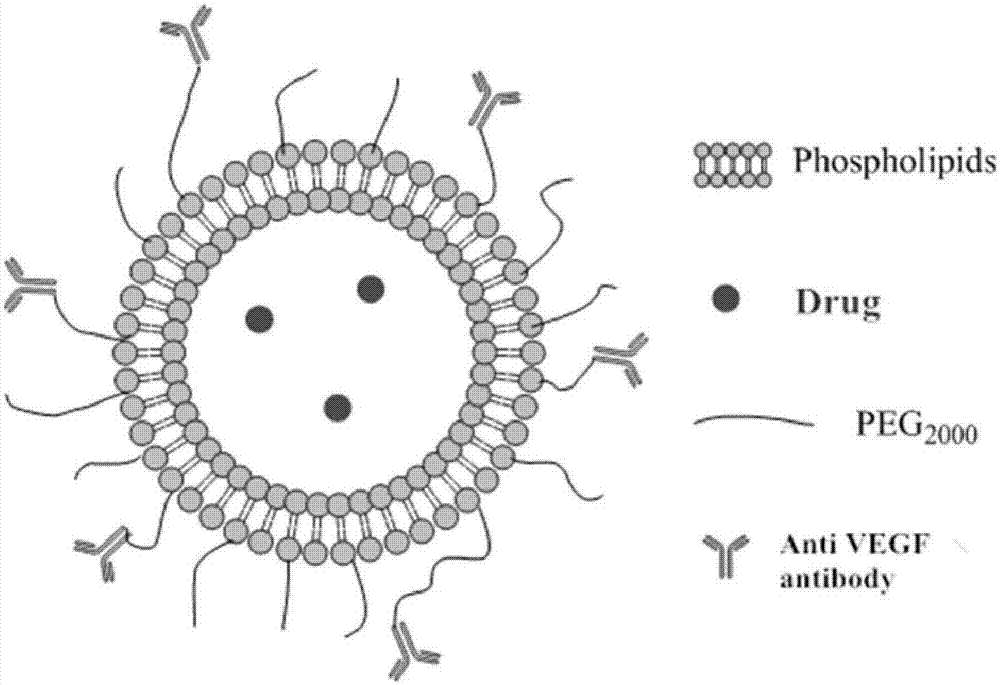
Antibody coupling type active targeting drug-loading long-circulating lipidosome and preparation method thereof
A long-circulation liposome and active targeting technology, which is applied in liposome delivery, antineoplastic drugs, pharmaceutical formulations, etc., can solve problems that have not yet been reported on oxaliplatin liposome research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Example 1, accurately weigh an appropriate amount of phospholipids, cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol 2000, phospholipids-polyethylene glycol 2000-maleimide (mass ratio is 4:1:1:0.4) Dissolve in chloroform, then weigh an appropriate amount of oxaliplatin raw material and dissolve in 5% glucose solution. Under the ultrasound of the probe, the solution dissolved in oxaliplatin was slowly added dropwise to chloroform to form a uniform and stable W / O emulsion, and the volume of aqueous phase: organic phase was 1:3. Then the chloroform was removed by rotary evaporation under reduced pressure at 45° C. to obtain a liposome solution, and then the liposome solution was freeze-thawed 3 times, and passed through a 0.2 μm carbonate membrane 8 times to obtain oxaliplatin-loaded long-circulation liposomes. The monoclonal antibody and 2-iminosulfane hydrochloride were mixed in an EP tube at a molar ratio of 1:200, placed on a low-speed shaker at room...
example 2
[0027] Example 2, accurately weigh an appropriate amount of phospholipids, cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol 2000, phospholipids-polyethylene glycol 2000-maleimide (mass ratio is 4:1:0.6:0.4 ) was dissolved in chloroform, and then an appropriate amount of oxaliplatin bulk drug was weighed and dissolved in 5% glucose solution. Under the ultrasound of the probe, the solution dissolved in oxaliplatin was slowly added dropwise to chloroform to form a uniform and stable W / O emulsion, and the volume of aqueous phase: organic phase was 1:3. Then the chloroform was removed by rotary evaporation under reduced pressure at 45°C to obtain a liposome solution, then the liposome solution was freeze-thawed 3 times, and passed through a 0.2 μm carbonate membrane 6 times to obtain the oxaliplatin-loaded long-circulation liposome. The monoclonal antibody and 2-iminosulfane hydrochloride were mixed in an EP tube at a molar ratio of 1:100, placed on a low-speed s...
example 3
[0028] Example 3, accurately weigh an appropriate amount of phospholipids, cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol 2000, phospholipids-polyethylene glycol 2000-maleimide and be dissolved in chloroform (mass ratio is 5:1: 0.8:0.4), then weigh an appropriate amount of oxaliplatin bulk drug and dissolve it in 5% glucose solution. Under the ultrasound of the probe, the solution dissolved in oxaliplatin was slowly added dropwise to chloroform to form a uniform and stable W / O emulsion, and the volume of aqueous phase: organic phase was 1:3. Then the chloroform was removed by rotary evaporation under reduced pressure at 45° C. to obtain a liposome solution, and then the liposome solution was freeze-thawed 3 times, and passed through a 0.2 μm carbonate membrane 8 times to obtain oxaliplatin-loaded long-circulation liposomes. The monoclonal antibody and 2-iminosulfane hydrochloride were mixed in an EP tube at a molar ratio of 1:200, placed on a low-speed sha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com