A kind of preparation method of aqueous phase chemiluminescent light source
A technology of chemiluminescence and hydrogen peroxide, which is applied in the field of chemiluminescence, can solve the problem that horseradish peroxidase is not suitable for chemiluminescence light sources, and achieve the effects of reducing poisoning incidents, economical preparation, and avoiding the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Synthetic Tris-Co (II) complex
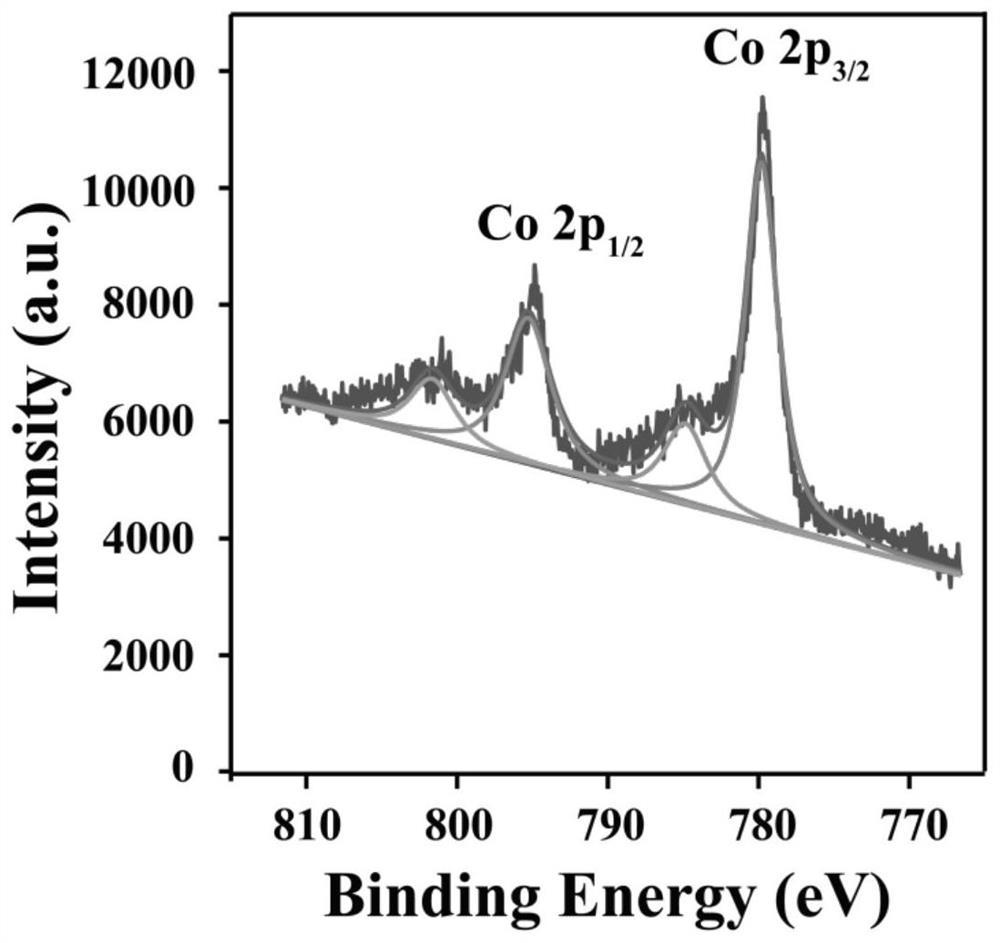
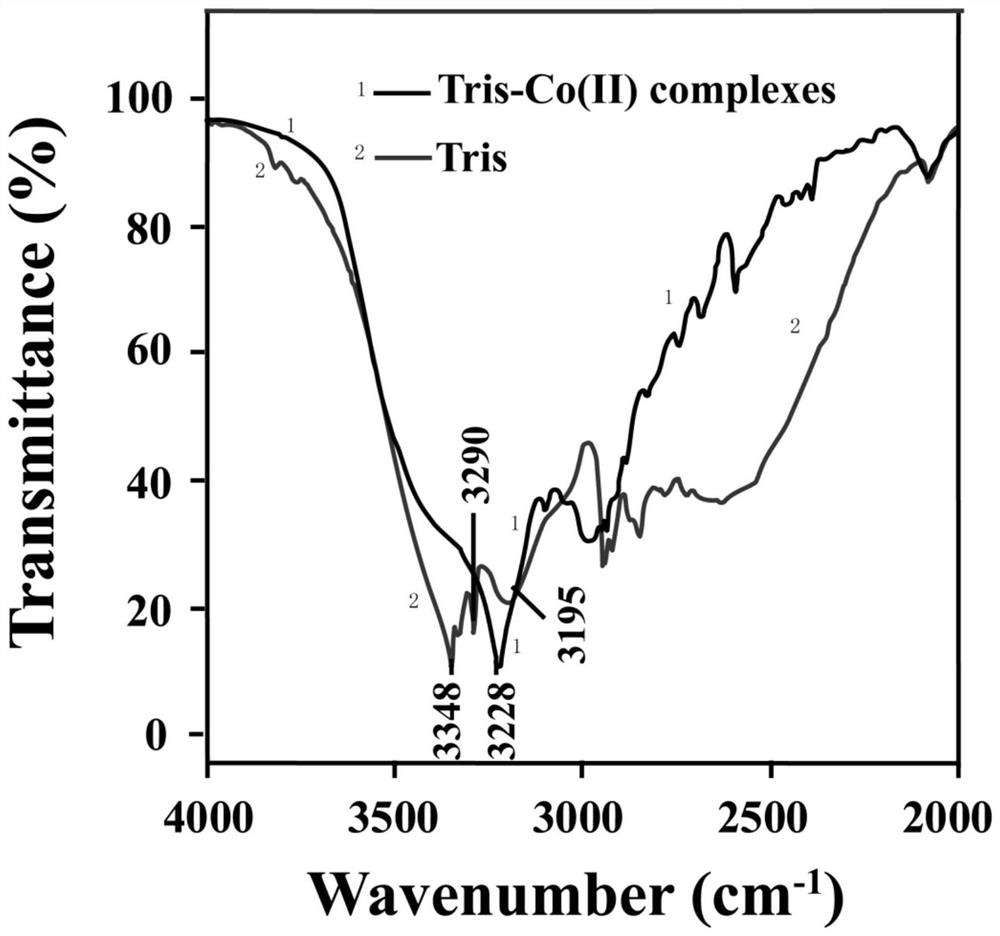
[0022] Add 0.2906g of Tris and 10mL of ultrapure water to a 50mL beaker and sonicate for 5 minutes to obtain a 240mM Tris aqueous solution; add 11.6mg of Co(NO 3 ) 2 ·6H 2 O and add ultrapure water to 50mL, and sonicate for 5 minutes to prepare a 0.8mM cobalt nitrate solution. Take 5mL Tris solution and 5mL Co(NO 3 ) 2 ·6H 2 O in a 50mL beaker, stirred at room temperature for 30 minutes to obtain a beige solution. Take 5 mL of the solution, centrifuge at 10,000 rpm for 5 minutes, remove the supernatant, and retain the beige solid precipitate. Add an appropriate amount of deionized water to wash, centrifuge again to remove the supernatant and retain the precipitate, and repeat twice. The obtained beige solid precipitate was placed in a vacuum oven at 25°C and dried for 4 hours to obtain a dry beige solid powder, which was pulverized with a mortar and then stored. Get 0.1g taupe powder and carry out X-ray energy spect...
Embodiment 2
[0023] Embodiment 2: Tris-Co (II) complex reacts with hydrogen peroxide in aqueous solution
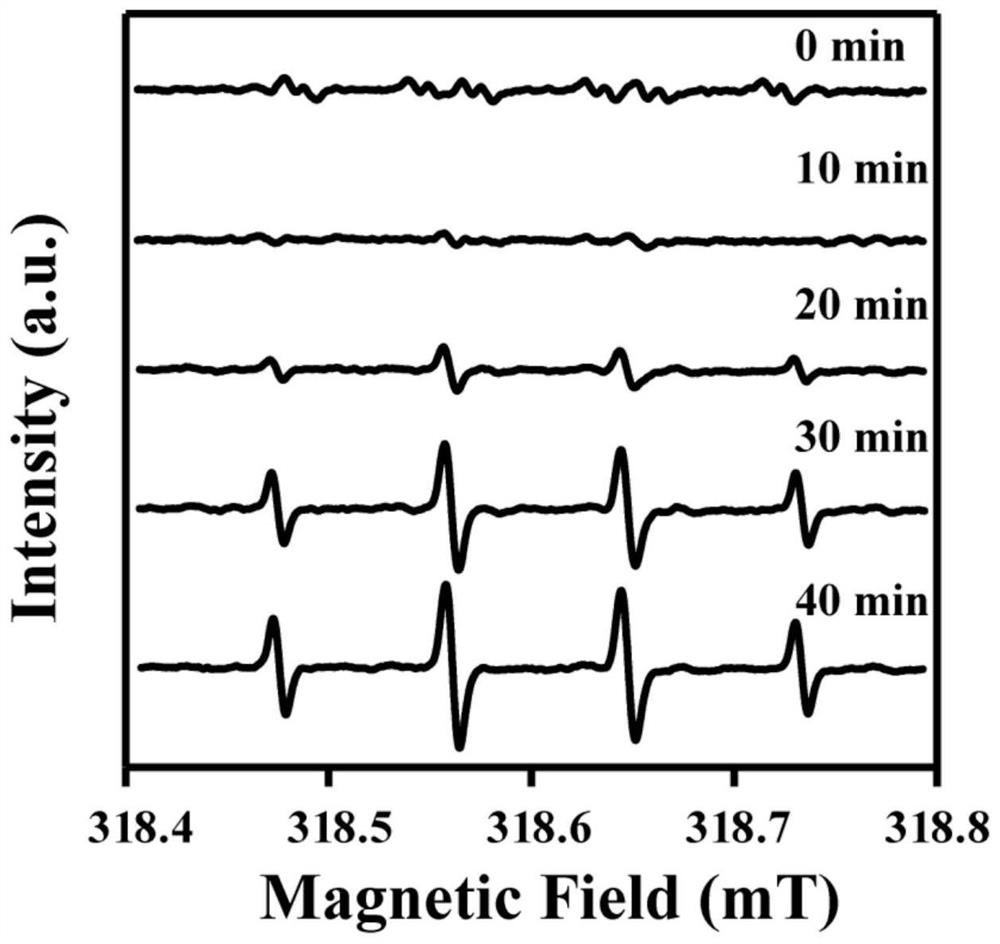
[0024] Take 900 μL Tris-Co(II) complex solution, put it in a 1.5 mL centrifuge tube, add 10 μL DMPO (hydroxyl radical scavenger), and then add 100 μL (50 mM) H 2 o 2 water solution to start the experiment. The reaction solution in the centrifuge tube was taken twice every 5min using a capillary tube and used for electron paramagnetic resonance (ESR) spectroscopy ( image 3 ) to test the content of hydroxyl radicals. After 50 minutes of testing and analyzing the data, we have proved the continuous generation of hydroxyl radicals in the system.
Embodiment 3
[0025] Embodiment 3: Contrast Luminol-Tris-Co(II)-H 2 o 2 Test method of chemiluminescence kinetic curve with traditional peroxalate system:
[0026] Put 100 μL of 10 mM luminol solution and 200 μL of Tris-Co(II) complex solution in a BPCL reaction bottle, and mix well. Adjust the BPCL operating voltage to -720V to ensure the stable operation of the BPCL ultra-weak chemiluminescence analyzer. Close the top cover of the BPCL detector and inject 100 μL of 20 mM H into the system 2 o 2 Aqueous solution, data collected and recorded by BPCL.
[0027] Comparative Test:
[0028] Put 100 μL of 10 mM CPPO solution, 100 μL of 40 μM BPEA solution, and 1M sodium salicylate solution in the BPCL reaction bottle, shake and mix, and adjust the operating voltage of BPCL to -720V. Close the top cover of the BPCL detector and inject 100 μL of 200 mM H into the system 2 o 2 Aqueous solution, data collected and recorded by BPCL.
[0029] The chemiluminescence kinetic curves of the two sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com