Huperzine A polymorphic substance and preparation method thereof, and medicinal composition
A technology for huperzine A and crystal forms, applied in the field of medicinal chemistry polymorph research, can solve the problems of cumbersome crystal form operations, unsuitable for industrial scale-up production, time-consuming and energy-consuming problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
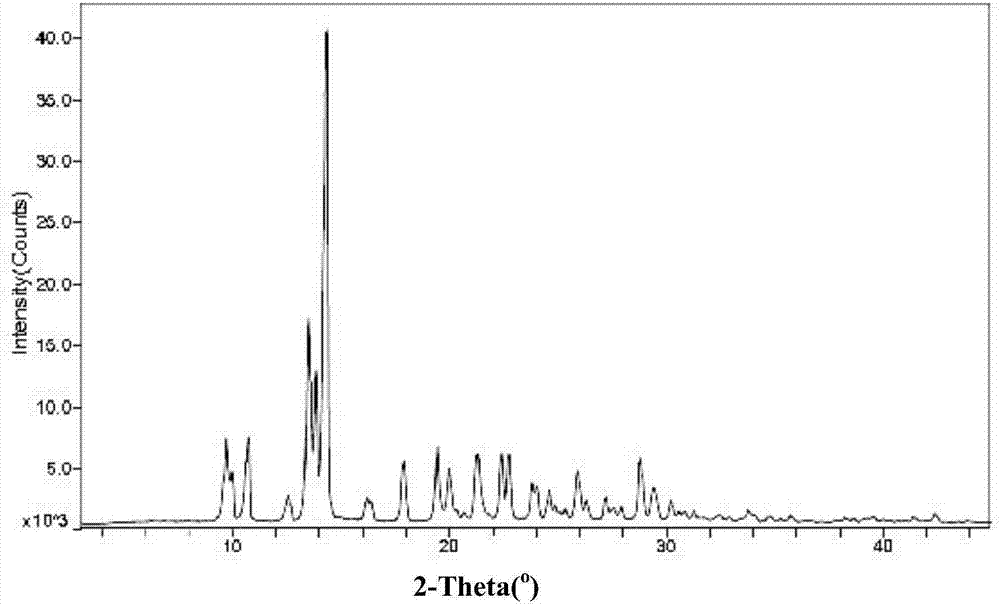
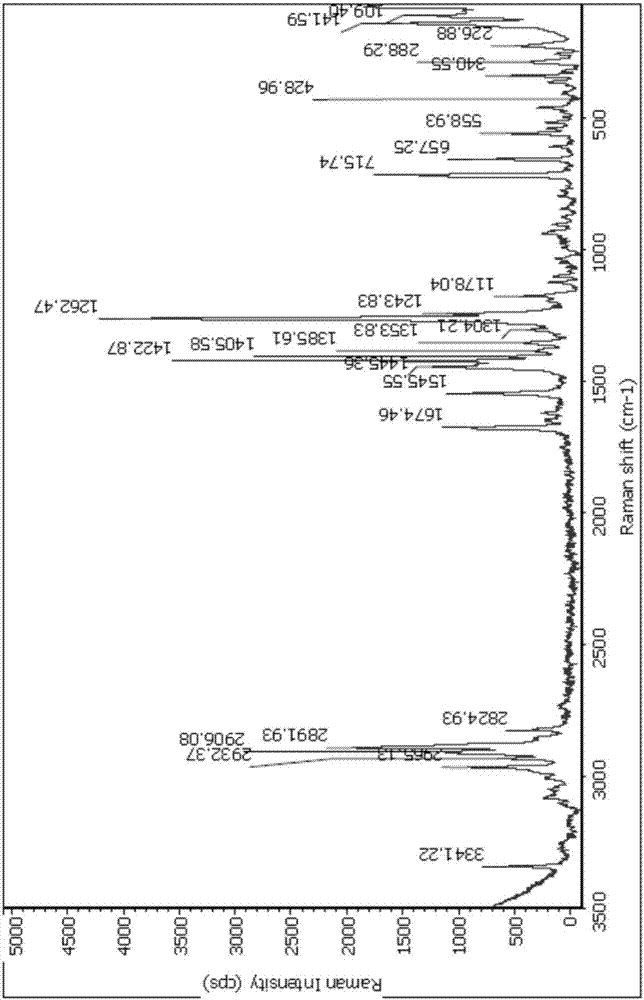
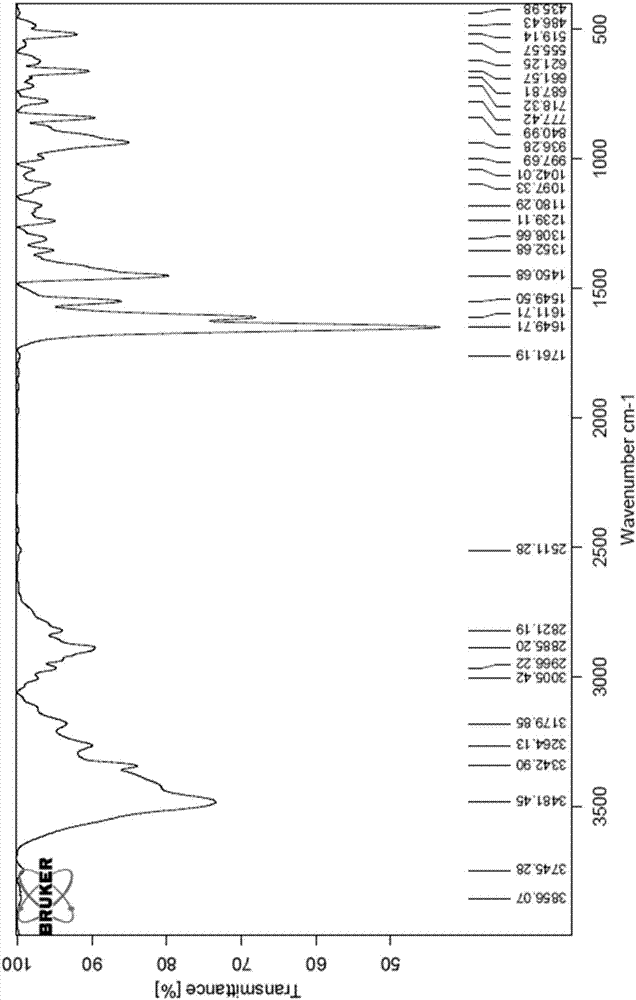
[0099] Add 1.0 g of Huperzine A amorphous powder into 1.4 mL of ethanol containing water (ethanol: water = 95V: 5V), heat to reflux until completely dissolved, continue to stir for 10 minutes, slowly cool to room temperature, continue to stir for 1 hour, filter , the filter cake was rinsed once with ice-containing ethanol, and the solid was vacuum-dried at 45° C. for 3 hours to obtain 0.7 g of a crystalline powder, which was determined by X-ray powder diffraction to be polymorph A. The specific peak positions are shown in Table 6 below:
[0100] The XRPD data of table 6 huperzine A polymorphic form A
[0101]
[0102]
Embodiment 2
[0104]Add 1.0 g of huperzine A amorphous powder into 1.4 mL of water-containing propanol (propanol: water = 95V: 5V), heat to reflux until completely dissolved, continue stirring for 10 minutes, slowly cool to room temperature, and continue stirring for 1 hour , filtered, and the filter cake was rinsed with ice-water isopropanol once, and the solid was vacuum-dried at 45°C for 3 hours to obtain 0.72 g of crystalline powder, which was determined by X-ray powder diffraction to be polymorphic a.
Embodiment 3
[0106] Add 1.0g of huperzine A amorphous powder into 1.4mL of water-containing isopropanol (isopropanol: water = 95V: 5V), heat and reflux until completely dissolved, continue stirring for 10 minutes, slowly cool to room temperature, and continue stirring Filter for 1 hour, rinse the filter cake with icy isopropanol once, and dry the solid under vacuum at 45°C for 3 hours to obtain 0.75 g of crystalline powder, which was determined by X-ray powder diffraction to be polycrystalline Model A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com