Establishment method of high-throughput drug screening model based on icam-1 signaling pathway
A technology of ICAM-1 and signaling pathway, which is applied in the field of high-throughput drug screening model establishment, and can solve problems such as adverse reactions and damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
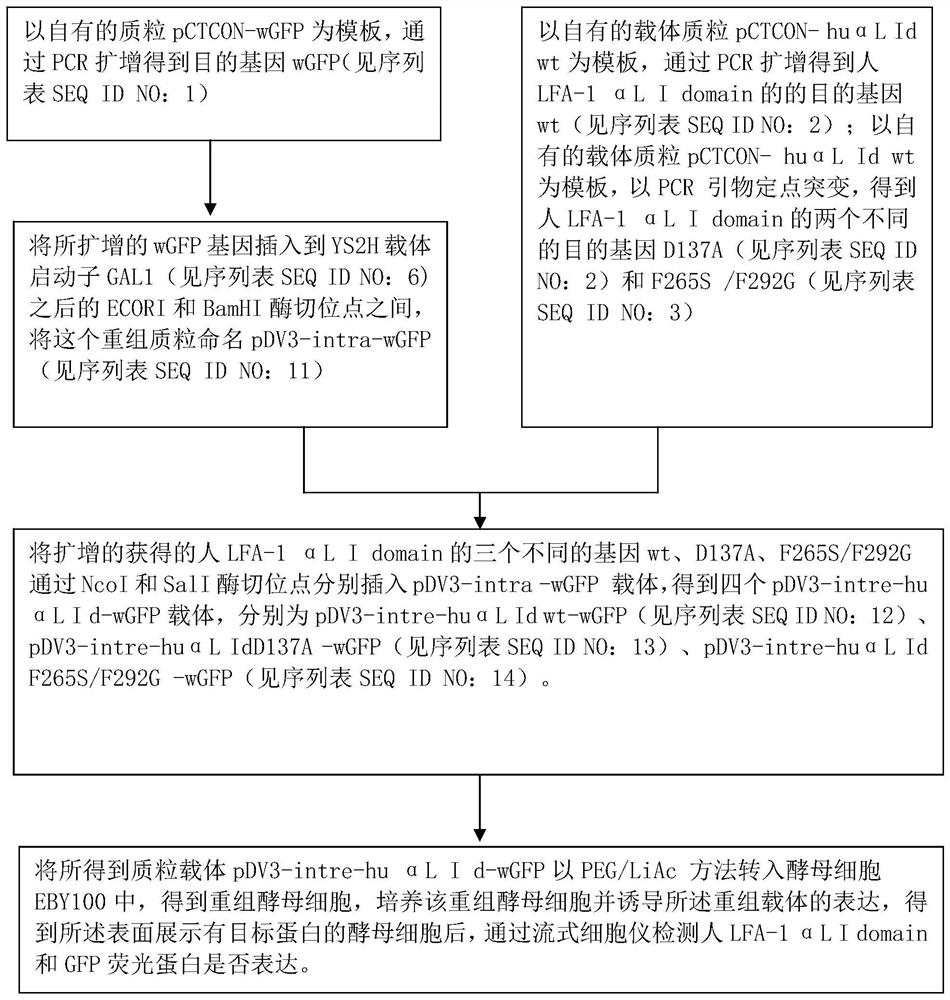
[0056] Example 1: Plasmid vector construction of engineered yeast
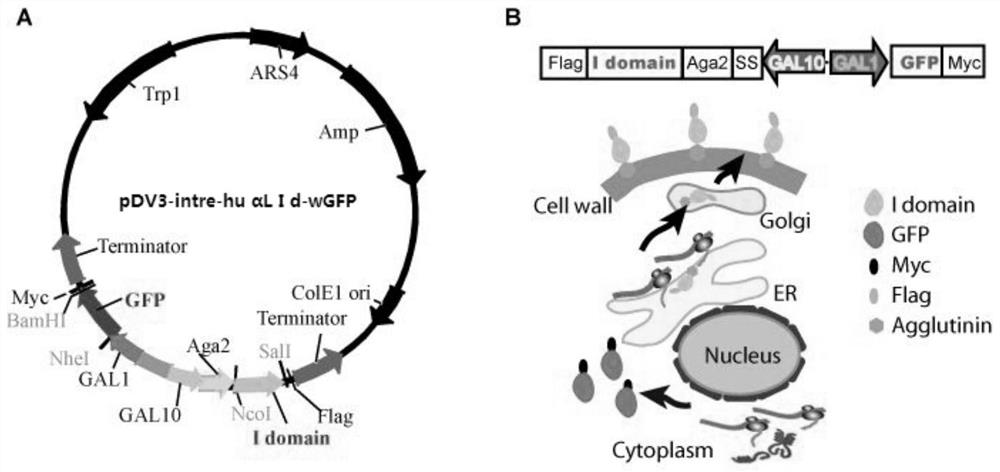
[0057] (1) Using your own plasmid pCTCON-wGFP as a template, use primers wGFP (5,-ATCGAATTCTACTTCATACATTTTCAAATTAAGATGGCTAGCGTGAGCAAGGGCGAGGAG-3, sequence-specific primers plus an ECORI site) and wGFP (5,-GTTCGGATCCAGTGATCCCGGCGGCGTTC-3 ,, sequence-specific primers plus BamHI site) to amplify the wGFP fragment (720bp). PCR reaction conditions: pre-denaturation at 94°C for 3 min; 35 cycles of 94°C for 30 sec, 54°C for 30 sec, and 72°C for 1 min; extension at 72°C for 10 min. The amplified wGFP gene PCR product was inserted between the ECORI and BamHI restriction sites behind the YS2H vector promoter GAL1, and positive clones were screened and sequenced to obtain the gene wGFP. We named this plasmid pDV3-intra-wGFP
[0058](2) On the basis of the above pDV3-intra-wGFP vector, the applicant inserted the human LFA-1αL I domain gene (wt, D137A, F265S / F292G) between the NcoI and SalI restriction sites. wt was ampl...
Embodiment 2
[0063] Example 2: Introduction of engineered yeast vector into yeast and expression of induced protein
[0064] The plasmid vector pDV3-intre-huαL Idwt of the above-mentioned three dual-channel protein expression engineering yeast of wGFP and LFA-1αL I domain gene (sequence see SEQ ID NO: 1 and SEQ ID NO: 2, 3, 4, 5) -wGFP, pDV3-intre-huαL Id D137A-wGFP and pDV3-intre-huαL Id F265S / F292G-wGFP with PEG / LiAc method (Ito H, Fukuda Y, Murata K, et al. Transformation of intact yeast cells treated with alkali cations [J].Journal of bacteriology,1983,153(1):163-168.) and a plasmid vector corresponding to a yeast cell were respectively introduced into the yeast cell EBY100 to obtain recombinant yeast cells, cultivate the recombinant yeast cells and induce the Expression of recombinant vector (specific steps follow: Hu X, Kang S, Chen X, et al. Yeast surface two-hybrid for quantitative in vivo detection of protein-protein interactions via the secretory pathway[J]. Journal of Biological...
Embodiment 3
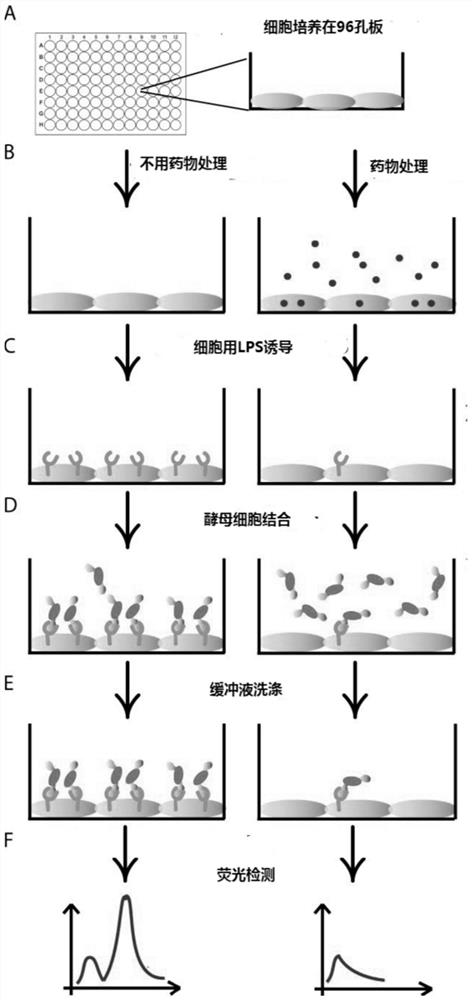
[0070] Example 3: Verification of protein expression in engineered yeast
[0071] After the recombinant yeast is induced, the expressions of I domain and GFP can be checked respectively by flow cytometry. The expression level of Idomain was detected with Flag tagged protein by flow cytometry. Specific steps are as follows:
[0072] (1) Aspirate 3 μl of yeast cells expressing wGFP and LFA-1αL I domain proteins into a 96-well V-shaped plate, take yeast cells that do not express proteins as a negative control, and add 100 μl of labeling buffer A pH=7.4 to each well (Recipe: PBS+0.5%BSA+10mM MgCl 2 ), mix well and centrifuge (4°C, 3min, 3000rpm) to pellet the cells, and remove the supernatant. Aspirate buffer A, add 20 μl of buffer A containing 10 μg / ml primary antibody (mouse anti-flag antibody (Santa Cruz Biotechnology, USA)) to each well, and incubate at 30°C for 30 minutes with shaking.
[0073] (2) Add 100 μl of buffer A to wash the yeast cells, centrifuge (4°C, 3min, 300...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com