Synthesis method of trenbolone acetate
A technology of trenbolone acetate and a synthetic method, which is applied in the field of androgen preparation, can solve problems such as ineffective technical and economical effects, and achieve the effects of optimizing reaction routes and processes, reducing raw material costs, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
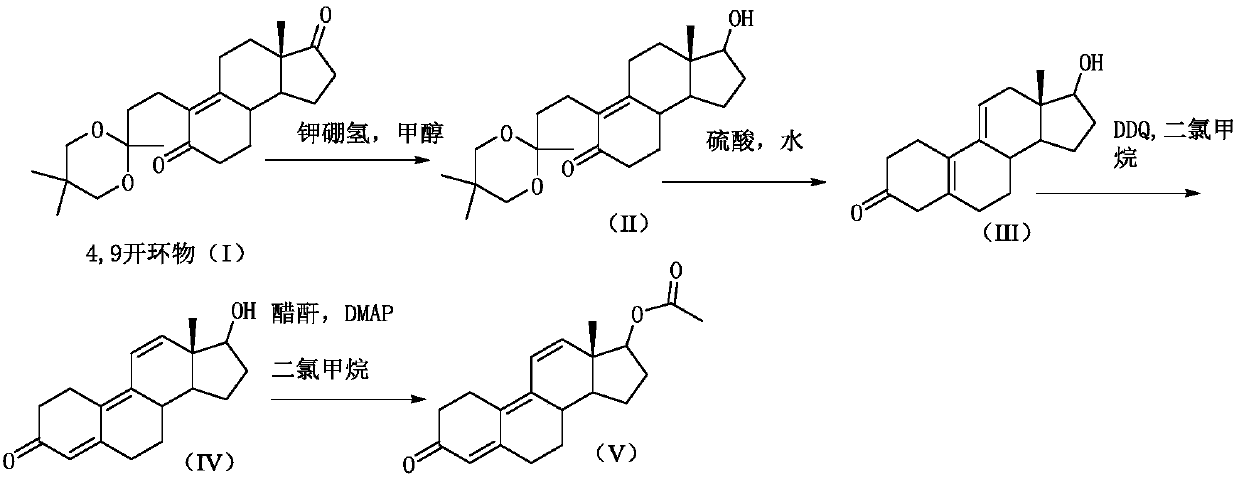
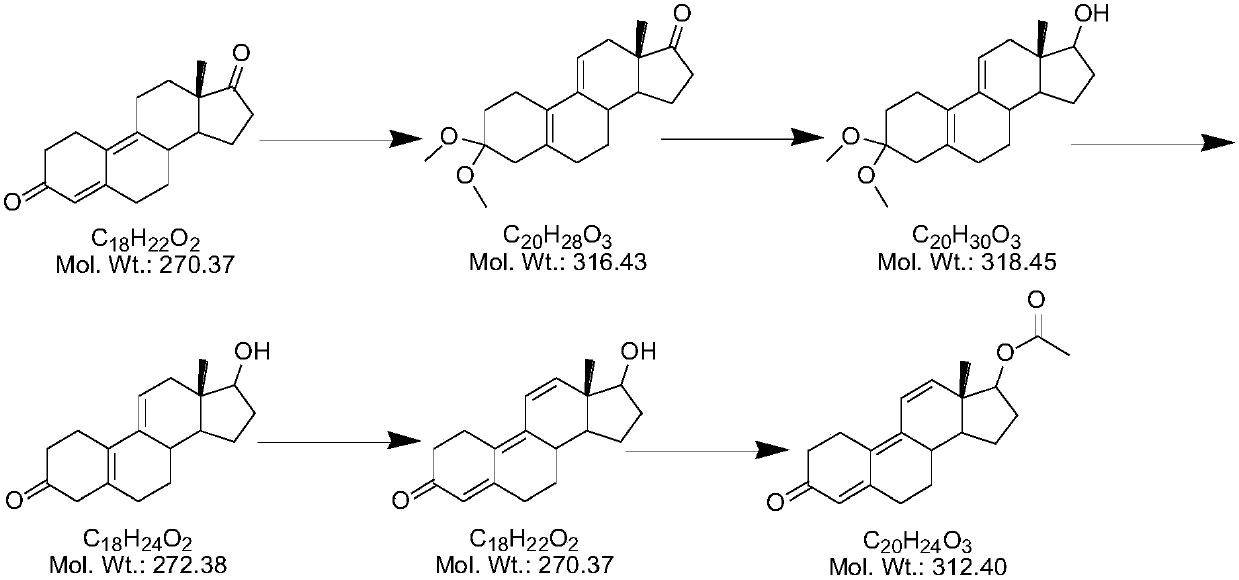
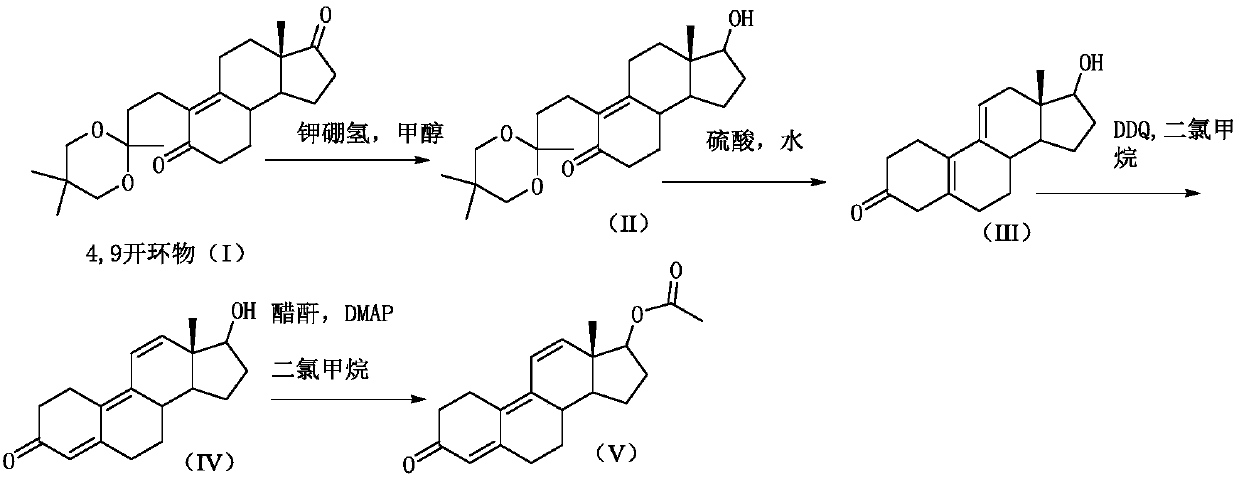
[0026] Step 1) reduction reaction:
[0027] 20kg of 4,9-ring-opened compound (I) was dissolved in 200kg of methanol, and 3kg of potassium borohydride was added in portions at 20-25°C. After the addition was completed, the reaction was incubated for 2 hours. TLC analysis of the reaction of the raw materials was complete to obtain compound (II).
[0028] Step 2) hydrolysis reaction
[0029] Add dilute acid (5.4kg sulfuric acid+25kg water) dropwise to the reaction system that has completed the reaction of step 1), neutralize to PH=7, prepare dilute sulfuric acid (7kg sulfuric acid+50kg water), prepare and cool to room temperature, and control the reaction system Temperature 20-25°C, add dilute sulfuric acid dropwise to the above reaction system, after dropping, stir for 2 hours, TLC analysis of raw material reaction is complete, add the reaction solution into sodium carbonate aqueous solution (14kg sodium carbonate + 140kg water) for water analysis, continue stirring 30min until...
Embodiment 2
[0035] Step 1) reduction reaction:
[0036] 20kg of 4,9-ring-opened compound (I) was dissolved in 300kg of methanol, and 5kg of potassium borohydride was added in portions at 20-25°C. After the addition was completed, the reaction was incubated for 2 hours. TLC analysis of the reaction of raw materials was complete to obtain compound (II).
[0037] Step 2) hydrolysis reaction
[0038] In the reaction system that has completed the reaction of step 1), add dropwise dilute acetic acid with a mass percentage concentration of 20% to neutralize to PH=7, prepare dilute sulfuric acid (8kg sulfuric acid+57kg water), prepare and cool to room temperature, and control the temperature of the reaction system at 20 ~25°C, add dilute sulfuric acid dropwise to the above reaction system, after dropping, stir the reaction for 2 hours, TLC analysis of raw material reaction is complete, add the reaction solution into sodium carbonate aqueous solution (15kg sodium carbonate + 150kg water) for water...
Embodiment 3
[0044] Step 1) reduction reaction:
[0045] 20kg of the 4,9-ring-opened compound (I) was dissolved in 160kg of methanol, and 3kg of potassium borohydride was added in portions at 20-25°C. After the addition was complete, the reaction was incubated for 2 hours. TLC analysis of the reaction of the raw materials was complete to obtain compound (II).
[0046] Step 2) hydrolysis reaction
[0047] Add dilute sulfuric acid with a mass percentage concentration of 15% to the reaction system that has completed the reaction of step 1) to neutralize to PH=7, and prepare dilute sulfuric acid (6kg sulfuric acid+44kg water) in addition, and cool it down to room temperature after mixing, and control the temperature of the reaction system 20~25°C, add dilute sulfuric acid dropwise to the above reaction system, after dropping, stir and react for 2 hours, TLC analysis of raw material reaction is complete, add the reaction solution into sodium carbonate aqueous solution (15kg sodium carbonate + 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com