Lithium ion batteries
一种锂离子电池、锂化的技术,应用在电池电极、二次电池、电路等方向,能够解决锂固定化电阻升高等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Sub-micron sized non-aggregated silicon particles in the form of fragments are produced by milling:
[0110] First, 500 g of pure silicon (particle size distribution: d10 = 8 μm, d50 = 15 μm and d90 = 25 μm; produced by grinding coarser particles with a fluidized bed jet mill) was uniformly suspended in 2 kg of ethanol (99% pure) by stirring for 20 minutes. )middle. The grinding space of a Netzsch LabStar LS1 laboratory stirred ball mill (with ZETA ceramic grinding system) was filled with 490 ml of yttria-stabilized zirconia grinding beads (average diameter: 0.3 mm) and closed. The suspension of silicon and ethanol was introduced into the cooled (15° C.) grinding vessel of the mill and pumped through the mill at a flux of 40 kg / h in circulation. Grinding was carried out for 55 minutes at a grinding speed of 3000 rpm. After the milling operation, ethanol was added to the suspension until its solids concentration was 21.8% by weight. The particle distribution was measu...
Embodiment 2
[0112] Production of anodes using the silicon particles of Example 1 and sodium carboxymethylcellulose as binder:
[0113] 11.0 g of the suspension of silicon in ethanol from Example 1 (solids concentration: 21.8 wt. %) into a solution of 12.52 g of 1.4% by weight sodium carboxymethylcellulose (Daicel, grade 1380) in water. After addition of 0.856 g of graphite (Imerys, KS6L C), the mixture was stirred for a further 30 minutes at a peripheral speed of 12 m / s. After degassing, the dispersion is applied to a copper foil (Schlenk Metallfolien, SE-Cu58) with a thickness of 0.030 mm by means of a film applicator (Erichsen, type 360) with a gap width of 0.20 mm. The prepared anode coating was then dried for 60 minutes at 80° C. and an air pressure of 1 bar. The average basis weight of the dry anodic coating is 2.90 mg / cm 2 .
Embodiment 3
[0114] Embodiment 3 (Ex.3):
[0115] Lithium ion battery with the anode of Example 2:
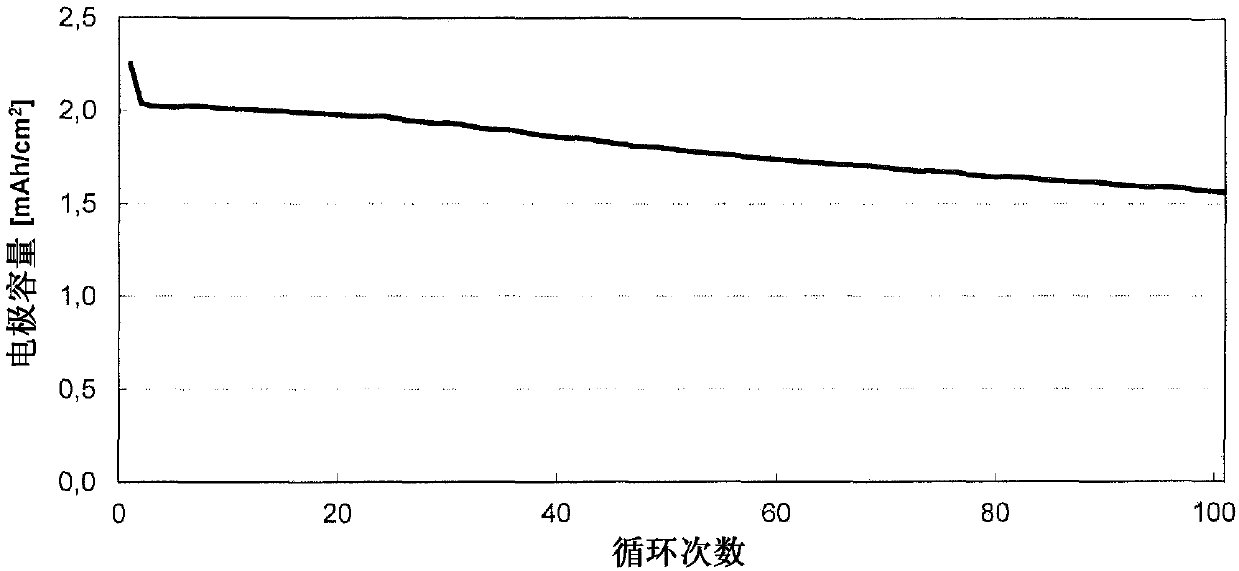
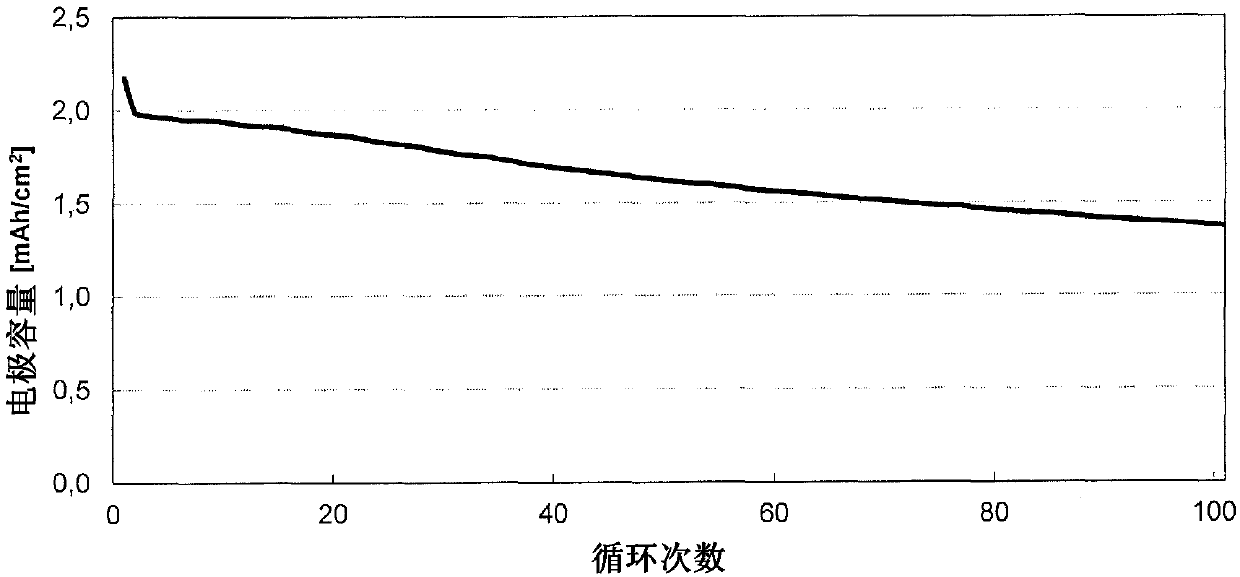
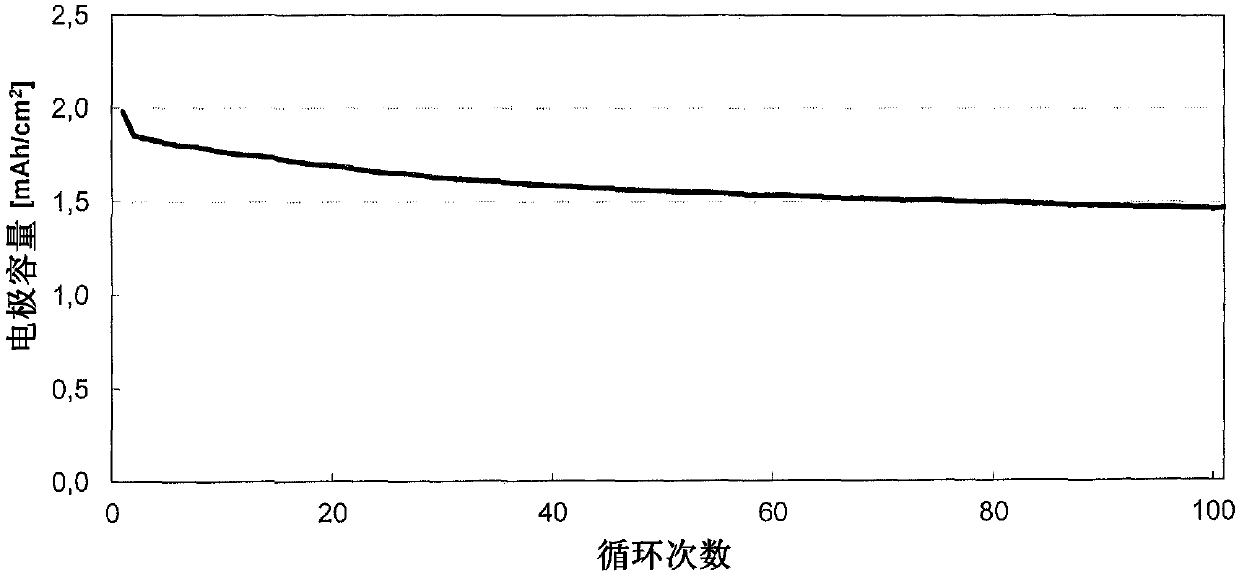
[0116] Electrochemical studies were performed in a coin cell (CR2032 type, Hohsen Corp.) employing a 2-electrode arrangement. The electrode coating from Example 2 was used as counter electrode or negative electrode (Dm = 15 mm) and would be based on 1:1:1 lithium nickel manganese cobalt oxide with a content of 94.0% and an average basis weight of 14.5 mg / cm 2Coatings (from Custom Cells) were used as working electrodes or positive electrodes (Dm = 15mm). Glass fiber filter paper (Whatman, GD Type D) impregnated with 120 μl of electrolyte was used as a separator (Dm = 16 mm). The electrolyte used consisted of a 1.0 molar solution of lithium hexafluorophosphate in a 3:7 (v / v) mixture of vinylene carbonate and diethyl carbonate to which 10.0% by weight of fluorodimethylsilylbutyronitrile and 2.0 % by weight tributylamine. In the glove box (2 O,O 2 ) to construct the battery; the water cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com