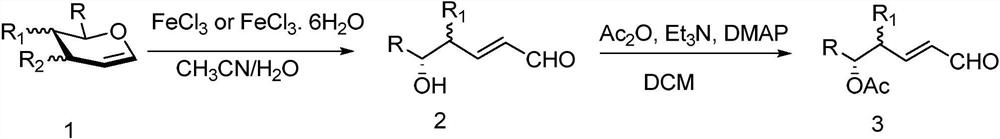
A kind of synthesis method of acetylated δ-hydroxyl-α, β-unsaturated furfural
A synthesis method and acetylation technology, applied in chemical instruments and methods, esterification saccharide, organic chemistry, etc., can solve the problems of harsh reaction conditions, high production cost, high price, etc., and achieve mild reaction conditions and non-toxic price. , the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 40.8mg (0.15mmol) of peracetylated glucosene, 20mL of acetone and 1mL of water into the flask, stir in an oil bath at 50°C, and then add 24.2mg (0.090mmol) of FeCl 3 ·6H 2 O catalyst, TLC monitors the reaction until the raw material is reacted, the reaction solution is cooled to room temperature, and water is added to quench the reaction, after being extracted with DCM, the organic phase is combined, and after being washed with saturated NaCl solution, it is dried and concentrated to obtain Perlin aldehyde, which is used in the ring-opening reaction Add 5 mL of DCM to the product, then add 31.4 μl triethylamine, 1.8 mg DMAP and 19 μl acetic anhydride in sequence, carry out acetylation reaction at 35 ° C, monitor the reaction by TLC until the reaction of the raw materials is complete, add saturated NH 4 The reaction was quenched with Cl solution, extracted with DCM, dried, and column chromatographed to obtain a pure product of 35.5 mg of Perlin glucuronide with an ac...
Embodiment 2
[0022] Add 32.1 mg (0.15 mmol) of fully acetylated rhamnose, 30 mL of dichloromethane and 1 mL of water into the flask, stir in an oil bath at a temperature of 25 ° C, and then add 2.5 mg (0.015 mmol) of FeCl 3 Catalyst, TLC monitors the reaction, until the raw material has reacted, after the reaction solution is cooled to room temperature, water is added to quench the reaction, after being extracted with DCM, the organic phase is combined, washed with saturated NaCl solution, dried and concentrated to obtain Perlin aldehyde, which is used in the ring-opening reaction Add 5mL DCM to the product, then add 25μl triethylamine, 5mg DMAP and 17μl acetic anhydride in sequence, carry out acetylation reaction at 0°C, monitor the reaction by TLC until the reaction of the raw materials is complete, add saturated NH 4 The reaction was quenched with Cl solution, extracted with DCM, dried, and column chromatographed to obtain 19.8 mg of Perlin rhamnoaldehyde with acetylated protecting group...
Embodiment 3
[0027] Add 30.0mg (0.15mmol) fully acetylated arabinose, 30mL acetonitrile and 0.5mL water into the flask, stir in an oil bath at 80°C, then add 15.1mg (0.09mmol) FeCl 3 Catalyst, TLC monitors the reaction, until the raw material has reacted, after the reaction solution is cooled to room temperature, water is added to quench the reaction, after being extracted with DCM, the organic phase is combined, washed with saturated NaCl solution, dried and concentrated to obtain Perlin aldehyde, which is used in the ring-opening reaction Add 5mL DCM to the product, then add 35μl triethylamine, 3mg DMAP and 19μl acetic anhydride in sequence, carry out acetylation reaction at 15°C, monitor the reaction by TLC until the reaction of the raw materials is complete, add saturated NH 4 The reaction was quenched with Cl solution, extracted with DCM, dried, and column chromatographed to obtain 26.4 mg of Perlin arabinfural, an acetylated protecting group, with a yield of 88%.
[0028] The product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com