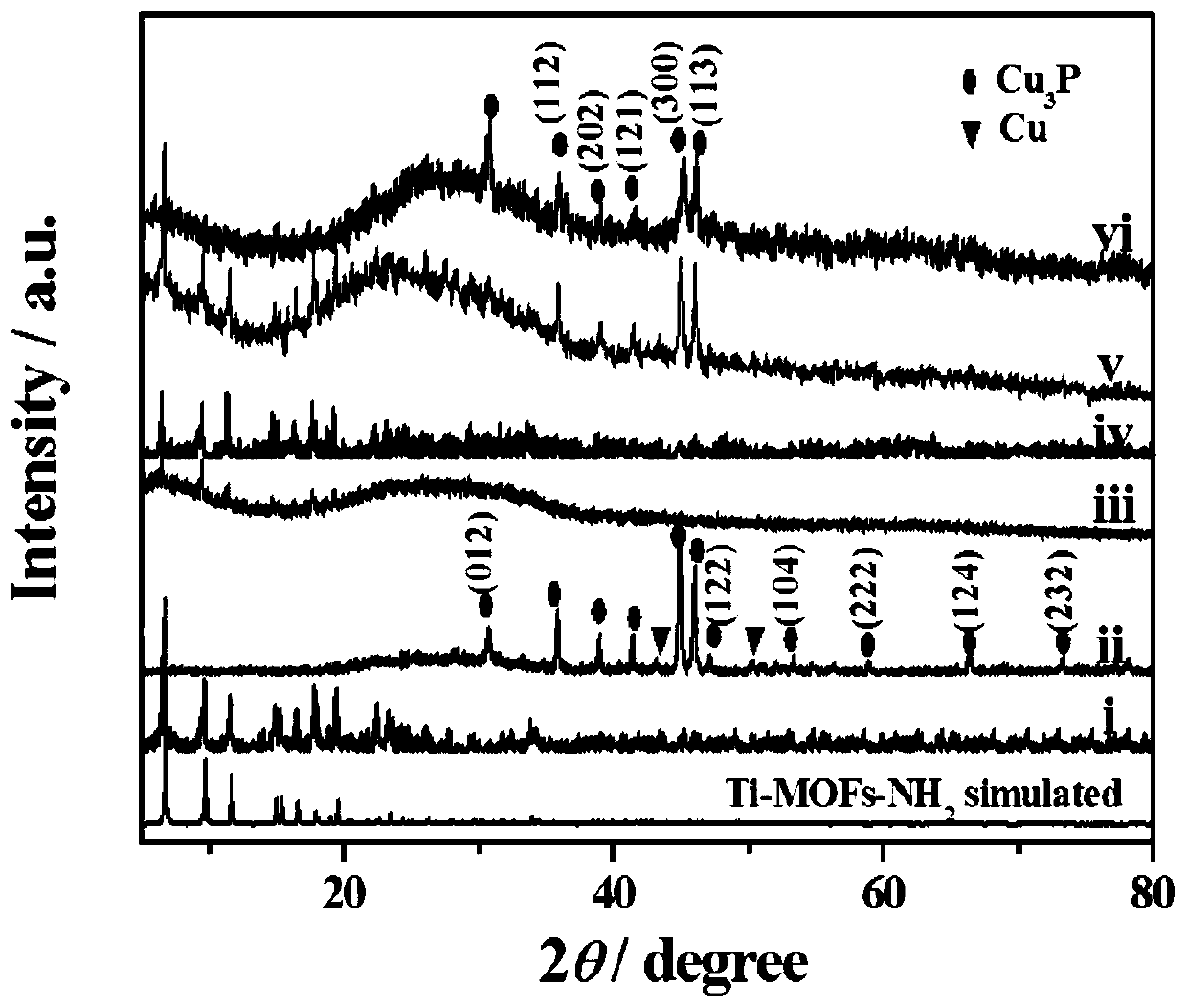
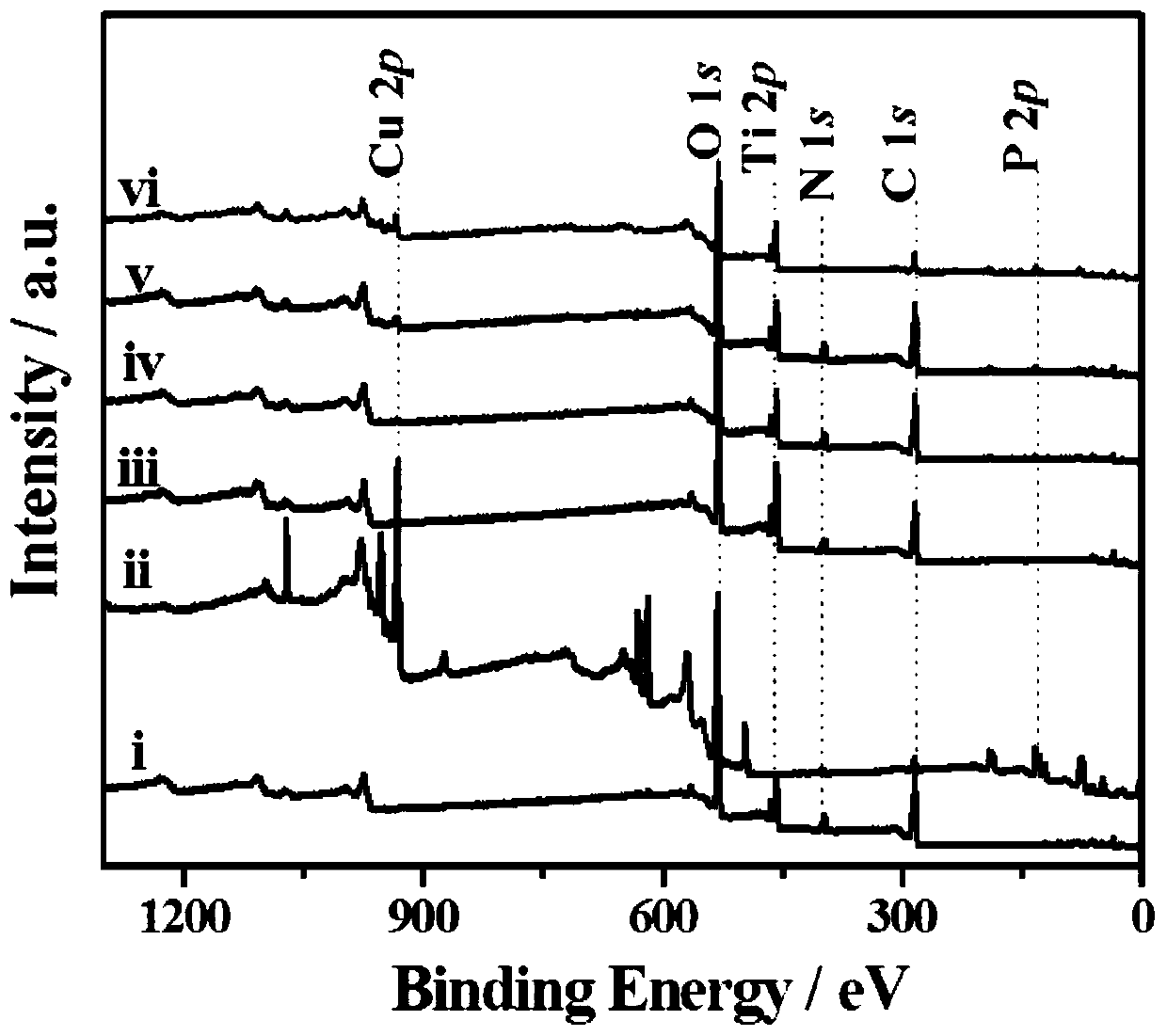
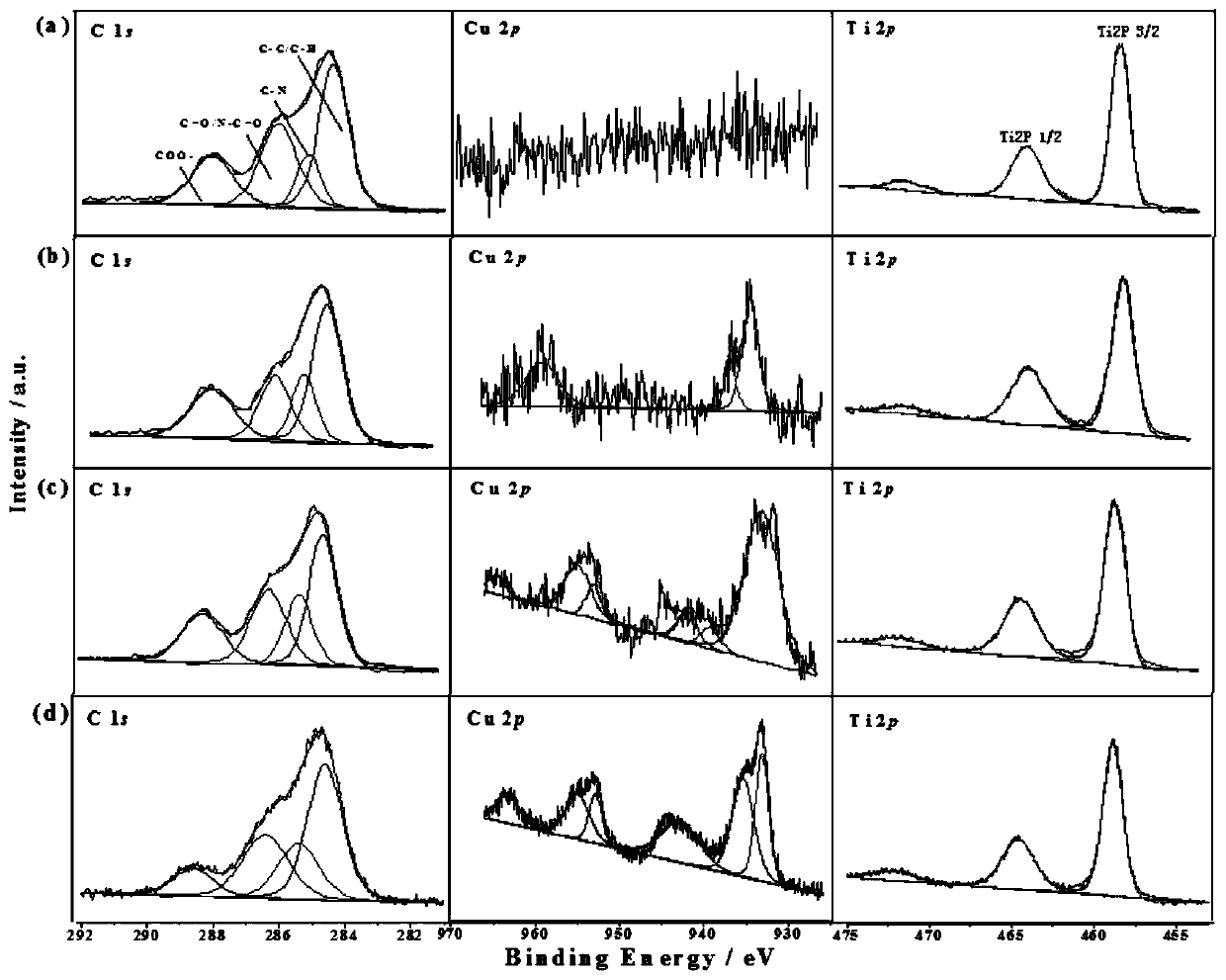
a cu 3 p@ti-mof-nh 2 Composite material, electrochemical sensor and its preparation method and application
A composite material and sensor technology, applied in the direction of material electrochemical variables, etc., can solve the problems of poor selectivity and low electrocatalytic activity, and achieve the effects of low manufacturing cost, simple process and huge application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Cu in this example 3 P@Ti-MOF-NH 2 Composite materials are prepared in the following steps:
[0046] 1) Cu 3 Preparation of P: Add excess 0.25mol / L NaOH solution to 50mL 0.05mol / L Cu(NO 3 ) 2 ·3H 2 O solution was reacted for 0.5h, the precipitate was centrifuged, washed several times with water, and dried in vacuum to obtain Cu(OH) 2 ;
[0047] 0.05g Cu(OH) 2 and 0.25g NaH 2 PO 2 Mix and grind evenly to obtain a mixture, and place the mixture in N 2 Atmosphere at 2°C·min -1 Heated at a heating rate of 300°C, kept the temperature for 2 hours, cooled naturally, washed the black solid with ultrapure water and ethanol several times, and dried it in a vacuum oven for later use;
[0048] 2) 50mg Cu 3 P was added to the methanol solution of PVP (0.35g PVP mixed with 10mL methanol), stirred at room temperature for 12h, centrifuged and washed with methanol three times, and the obtained solid phase was dispersed in 3.6mL DMF to obtain Cu 3 P dispersion;
[0049] Add...
Embodiment 2
[0055] Cu in this example 3 P@Ti-MOF-NH 2 Composite materials are prepared in the following steps:
[0056] 1) Cu 3 The preparation of P is with embodiment 1;
[0057] 2) 5mg Cu 3 P was added to the methanol solution of PVP (0.35g PVP mixed with 10mL methanol), stirred at room temperature for 12h, centrifuged and washed with methanol three times, and the obtained solid phase was dispersed in 3.6mL DMF to obtain Cu 3 P dispersion;
[0058] Add 0.4mL methanol, 0.22g 2-aminoterephthalic acid and 0.24mL tetrabutyl titanate into the beaker, stir magnetically for 5min to form a mixed solution;
[0059] Will Cu 3 After the P dispersion and the mixed solution were mixed, they were transferred to a polytetrafluoroethylene-lined reactor and reacted at 150 °C for 48 h. The solid matter was collected by centrifugation, washed three times with methanol, and dried to obtain Cu 3 P@Ti-MOF-NH 2 -5.
[0060] The electrochemical sensor of this embodiment is prepared by referring to the...
Embodiment 3
[0062] Cu in this example 3 P@Ti-MOF-NH 2 Composite materials are prepared in the following steps:
[0063] 1) Cu 3 The preparation of P is with embodiment 1;
[0064] 2) 20mg Cu 3 P was added to the methanol solution of PVP (0.35g PVP mixed with 10mL methanol), stirred at room temperature for 12h, centrifuged and washed with methanol three times, and the obtained solid phase was dispersed in 3.6mL DMF to obtain Cu 3 P dispersion;
[0065] Add 0.4mL methanol, 0.22g 2-aminoterephthalic acid and 0.24mL tetrabutyl titanate into the beaker, stir magnetically for 5min to form a mixed solution;
[0066] Will Cu 3 After the P dispersion and the mixed solution were mixed, they were transferred to a polytetrafluoroethylene-lined reactor and reacted at 150 °C for 48 h. The solid matter was collected by centrifugation, washed three times with methanol, and dried to obtain Cu 3 P@Ti-MOF-NH 2 -20.
[0067] The electrochemical sensor of this embodiment is prepared by referring to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com