Efficient synthesizing method of estriol
A synthesis method and technology of estriol, applied in the direction of steroids, organic chemistry, etc., can solve the problems of easy isomers, etc., and achieve the effects of fast reaction speed, high solid purity, and improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
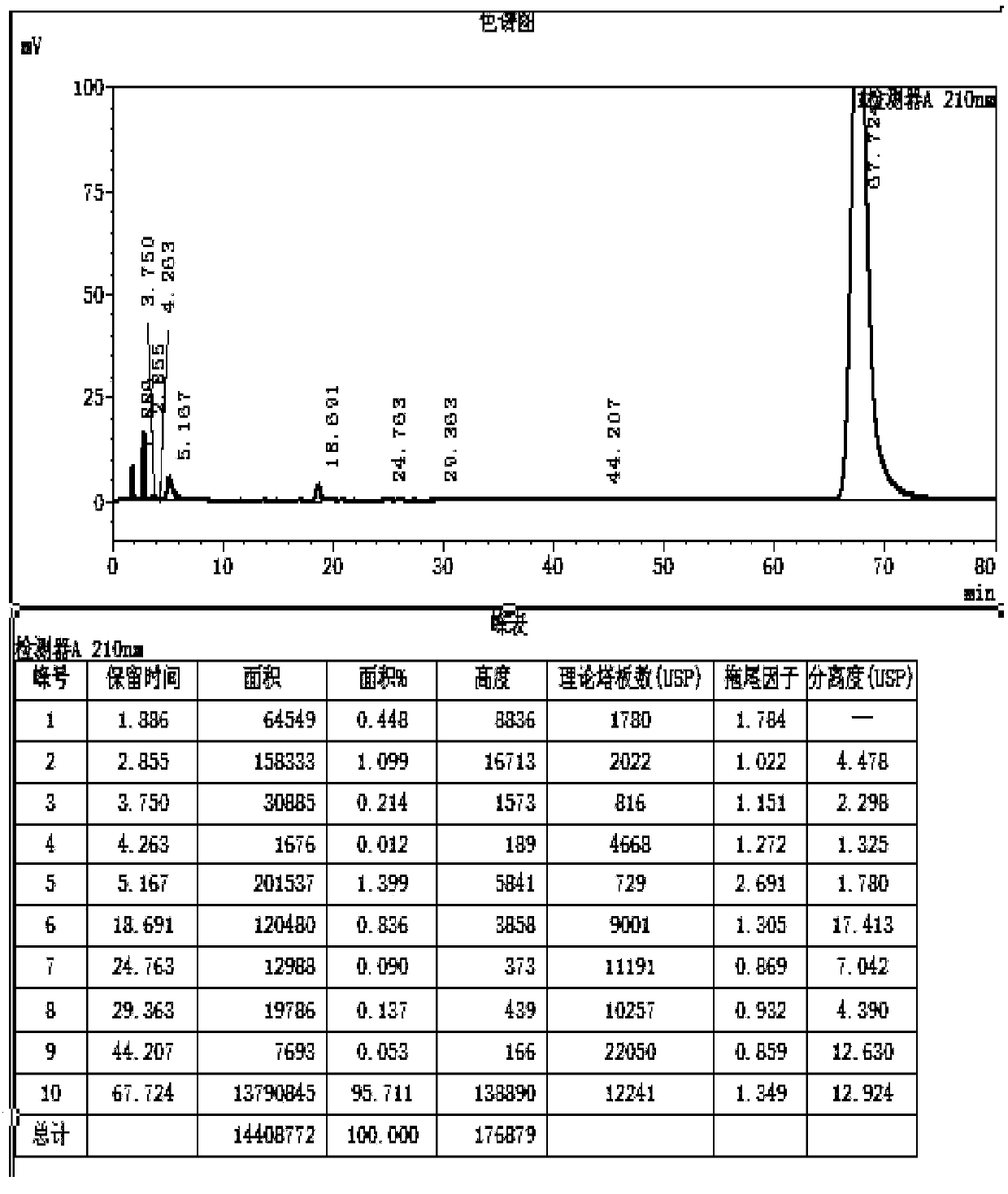
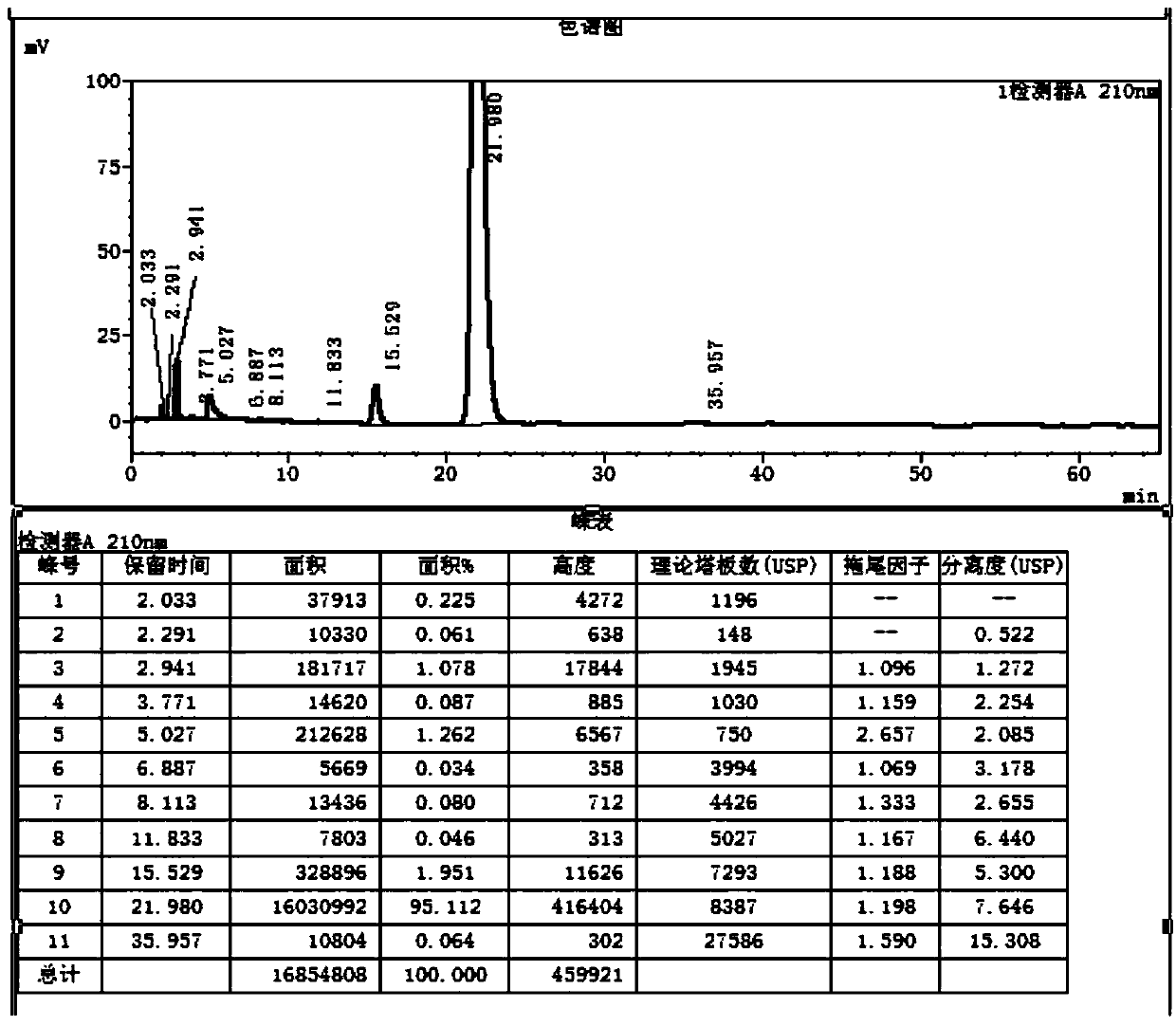
Image
Examples
Embodiment 1
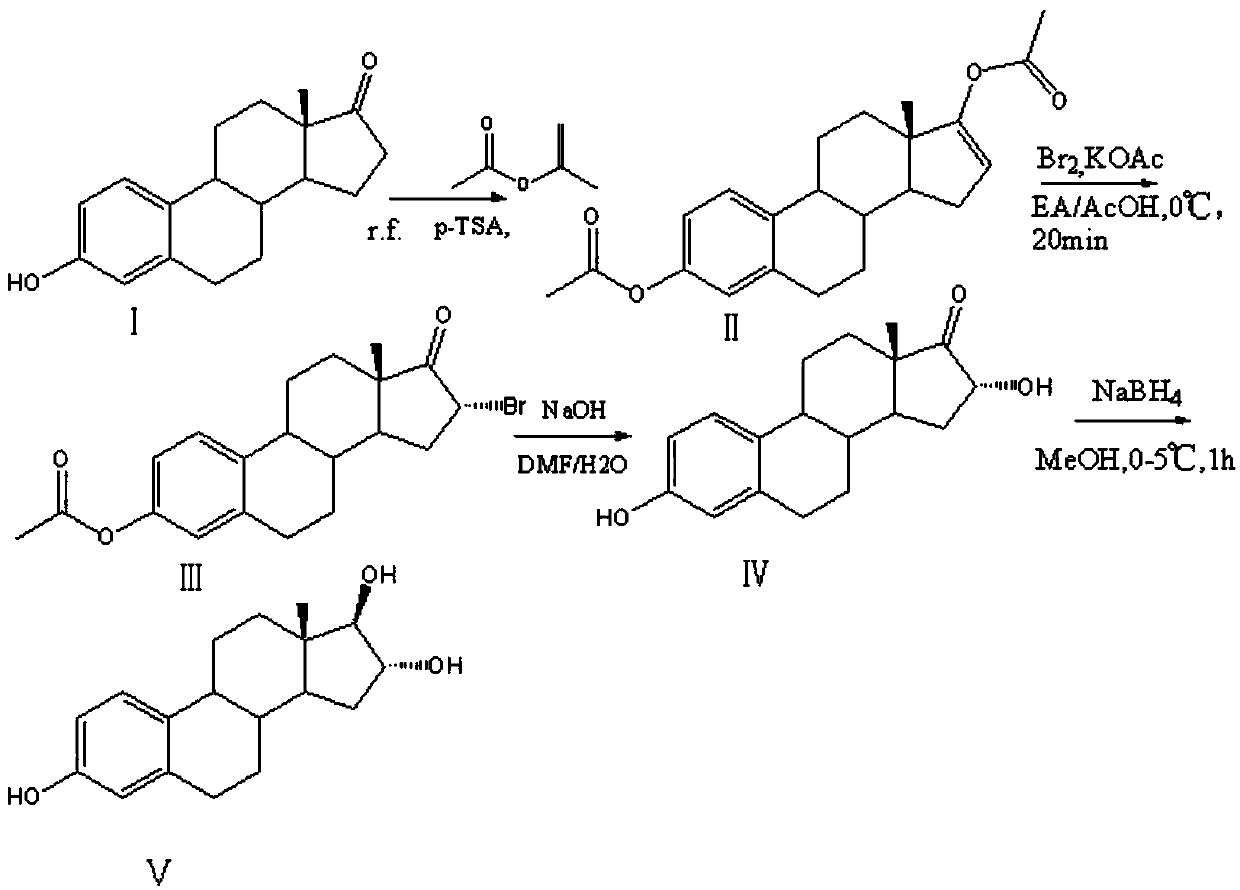
[0025] Such as figure 1 The efficient synthetic method of described estriol comprises the following steps:
[0026] S1. Add 20g of estrone, 60ml of isopropenyl acetate, and 5g of p-toluenesulfonic acid into the reaction vessel, heat up to reflux for 3 hours, recover 25ml of isopropenyl acetate, then add 20ml of isopropenyl acetate, and continue to keep warm for 2-3 hours , repeatedly recovering part of isopropenyl acetate, and then adding isopropenyl acetate to continue the reaction until no obvious monoester residues were detected by TLC, cooling, adding triethanolamine to adjust pH=9, and suction filtration to obtain brominated reaction intermediates.
[0027] S2. Take 10g of the intermediate to be brominated in the reaction container, add 200ml of ethyl acetate, 3.22g of sodium acetate and 20ml of acetic acid, add 150g of liquid bromine and carry out the bromination reaction at 5°C for 1.5h, and TLC detects that the reaction of the raw materials is complete , Add water to...
Embodiment 2
[0033] Such as figure 1 The efficient synthetic method of described estriol comprises the following steps:
[0034] S1. Add 20g of estrone, 60ml of isopropenyl acetate, and 5g of p-toluenesulfonic acid into the reaction vessel, heat up to reflux for 1 hour, recover 25ml of isopropenyl acetate, then add 20ml of isopropenyl acetate, continue to keep warm for 2 hours, repeat Recover part of isopropenyl acetate, then add isopropenyl acetate to continue the operation of the reaction until TLC detects that there is no obvious monoester compound residue, cool down, add triethanolamine to adjust pH=8, and filter with suction to obtain the product to be brominated. intermediate.
[0035] S2. Take 10g of the intermediate to be brominated in the reaction vessel, add 200ml of ethyl acetate, 3.22g of sodium acetate and 20ml of acetic acid, add 90g of liquid bromine and carry out the bromination reaction at -5°C for 0.5h, and detect the reaction of the raw materials by TLC Completely, aft...
Embodiment 3
[0041] Such as figure 1 The efficient synthetic method of described estriol comprises the following steps:
[0042]S1. Add 20g of estrone, 60ml of isopropenyl acetate, and 5g of p-toluenesulfonic acid into the reaction vessel, heat up to reflux for 2 hours, recover 25ml of isopropenyl acetate, then add 20ml of isopropenyl acetate, and continue to insulate for 2.5 hours. Repeat the operation of recovering part of isopropenyl acetate, and then add isopropenyl acetate to continue the reaction. After TLC detects that there is no obvious monoester residue, cool down, add triethanolamine to adjust pH=8.5, and filter with suction to obtain the bromination reaction intermediates.
[0043] S2. Take 10g of the intermediate to be brominated in the reaction container, add 200ml of ethyl acetate, 3.22g of sodium acetate and 20ml of acetic acid, add 125g of liquid bromine and carry out the bromination reaction at -0°C for 1 hour, and TLC detects that the reaction of the raw materials is co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com