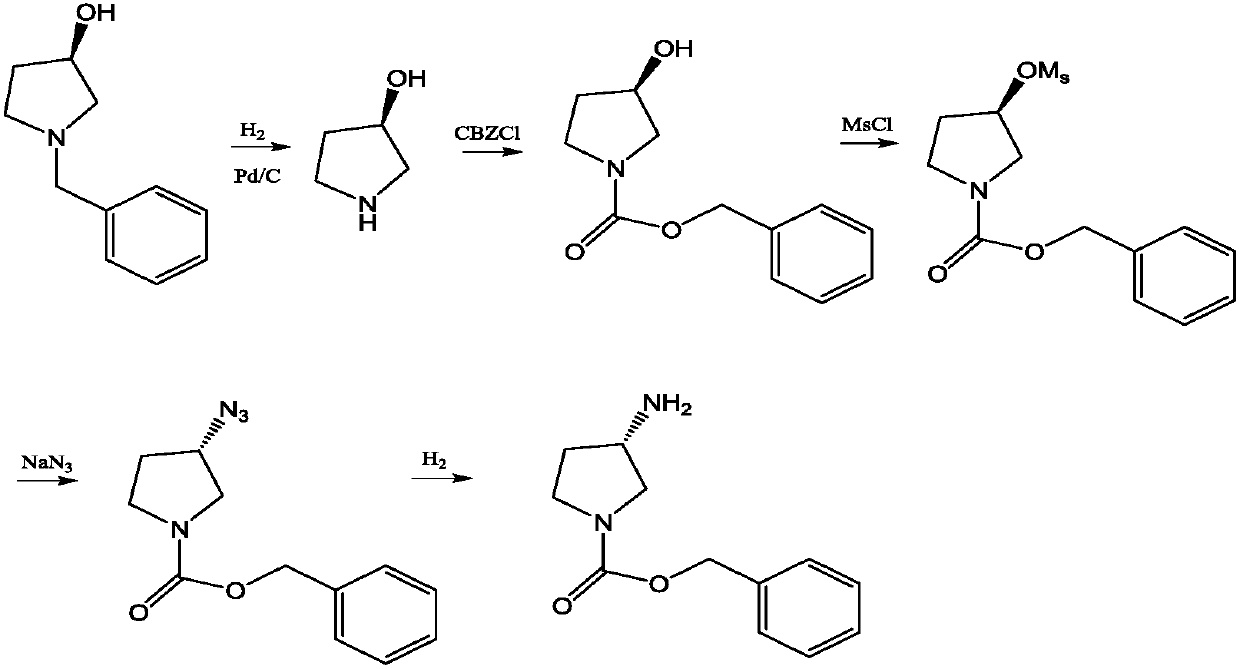
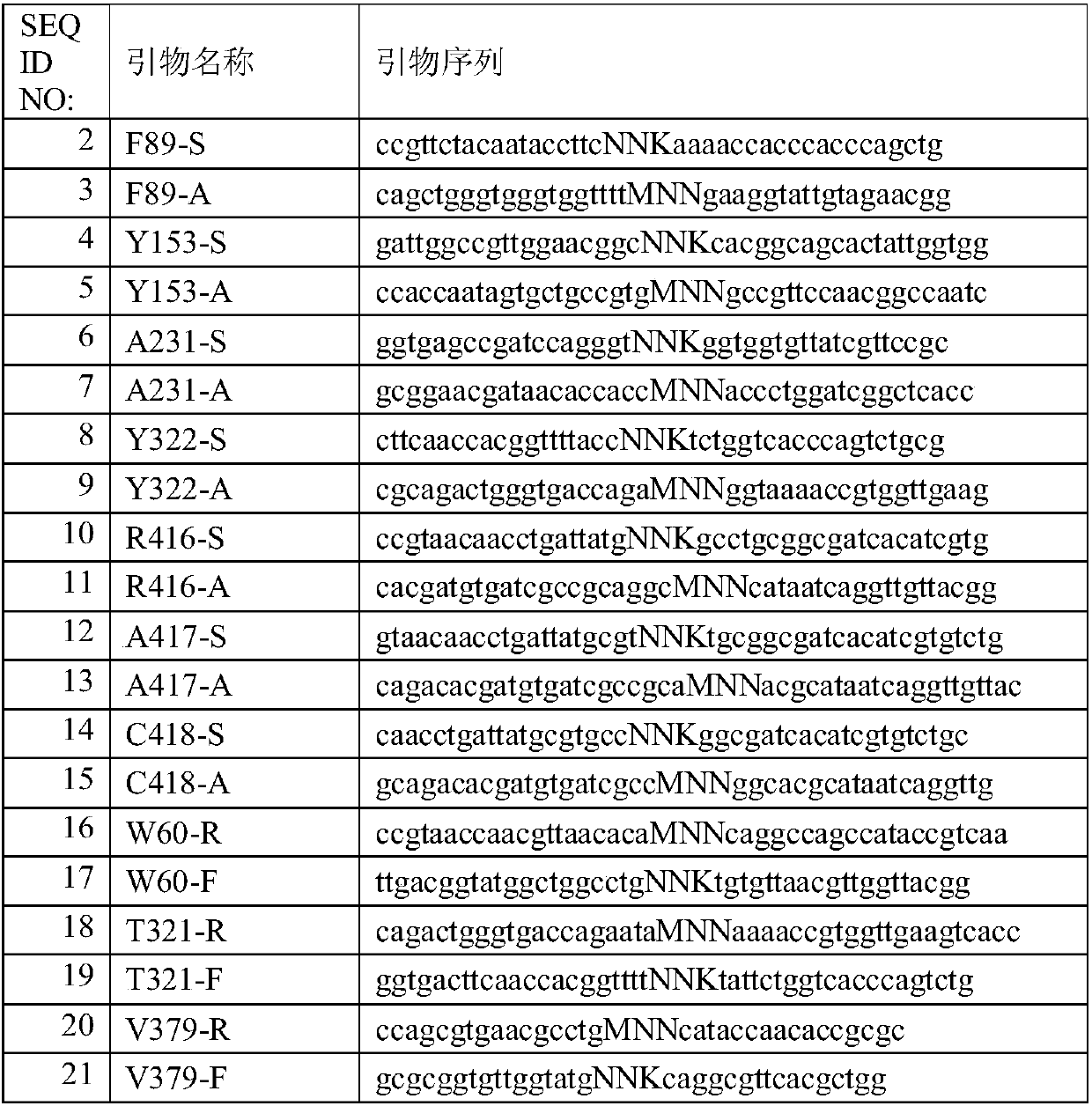
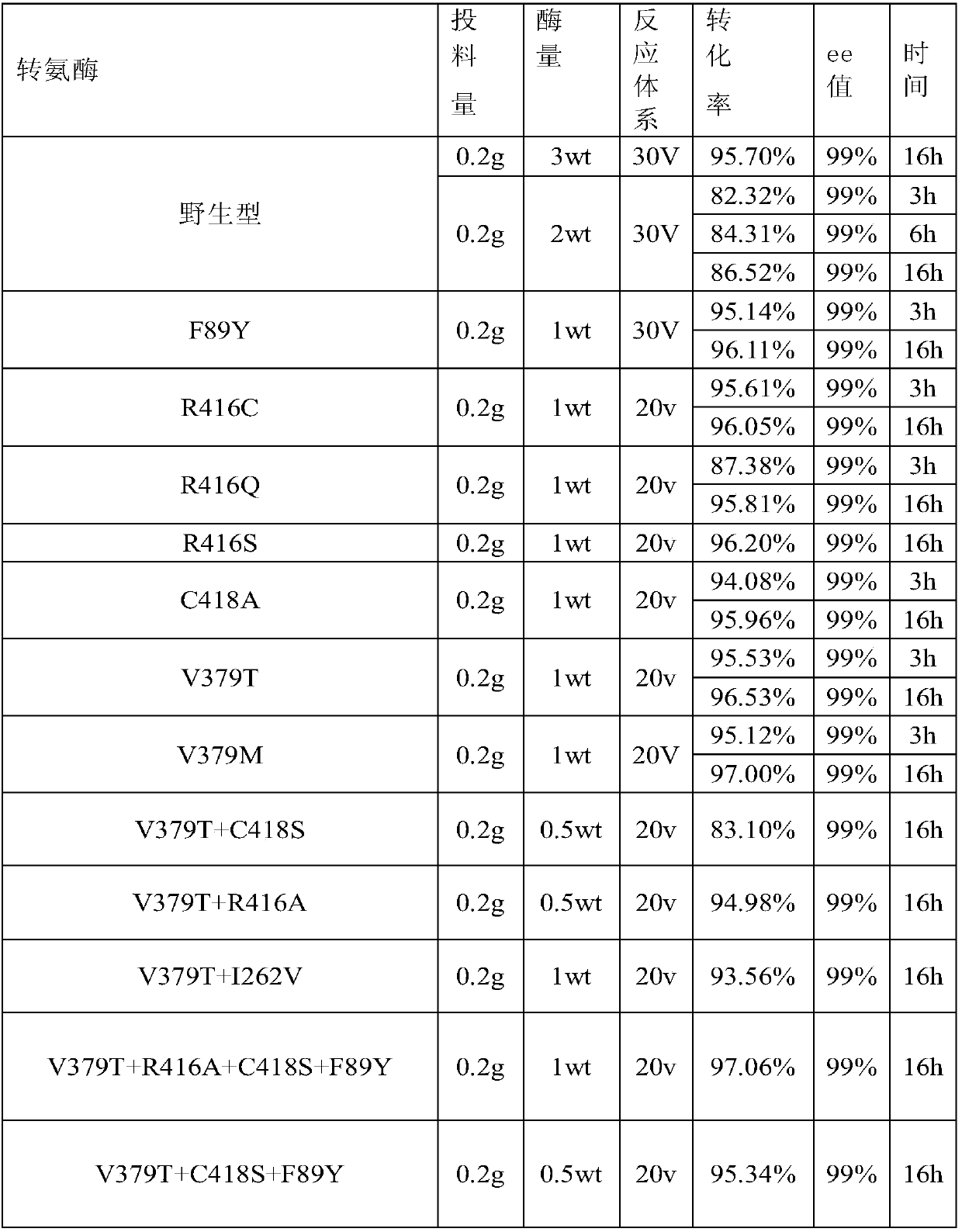
Transaminase mutant and application thereof
A transaminase and mutant technology, applied in the field of enzyme engineering, can solve the problems of increased production batches and production costs, insufficient catalytic activity of enzymes, large amount of organic solvents, etc., to reduce usage, production batches and material costs The effect of reducing usage and industrial production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Using 36 pairs of site-directed mutagenesis primers designed on the QuikChange Primer Design web page and 9 saturation mutagenesis primers shown in Table 1, complete linear fragments were obtained by whole plasmid PCR, and the above PCR products were digested with DpnI to remove the parent template of the starting gene. It was transformed into Escherichia coli BL21 (DE3), spread on LB petri dishes containing 50 μg / ml ampicillin, and cultured at 37°C overnight. Saturation mutations were screened by high-throughput. Specifically, the following methods were used for high-throughput screening of the above mutants:
[0089] (1) Induced expression in 96-well plate: pick a single clone and inoculate it in LB liquid medium containing 100 μg / ml ampicillin, and culture it with shaking at 37°C to OD 600 = 0.6, IPTG was added to a final concentration of 0.2 mM, and expression was induced overnight at 25°C.
[0090] (2) Enzyme solution preparation method: centrifuge the 96-well pl...
Embodiment 2
[0091] Example 2: Activity test of transaminase mutants on N-Cbz-3-pyrrolidone substrates
[0092] The above mutants with higher enzyme activity than wild-type transaminase were inoculated into 500 ml of LB liquid medium containing 100 μg / ml ampicillin, and cultured with shaking at 37°C to OD. 600 = 0.6, IPTG was added to a final concentration of 0.2 mM, and expression was induced at 25°C. After 16h induction, the cells were collected by centrifugation at 6000g for 10min. The cells were disrupted with an ultrasonic disrupter (JY92-2D, Ningbo Xinzhi Biotechnology Co., Ltd.), and the supernatant was obtained by centrifugation at 10,000 g for 20 min at 4°C for activity detection.
[0093] Dissolve 0.2g N-Cbz-3-pyrrolidone substrate in 0.5ml DMSO and mix well, add 1.25ml of 4.3M isopropylamine hydrochloride, 0.2ml of 0.01g / ml PLP, and 0.3~2wt recombinant crude enzyme, The reaction system was supplemented at 20-30 V with 100 mM phosphate buffer pH 7.0, adjusted to pH 7.0, and rea...
Embodiment 3
[0099] The 33 transaminases induced by mutants numbered 1 to 21, 33 to 43 and 54 were used for the screening of the synthesis reaction of (S)-1-benzyloxycarbonyl-3-aminopiperidine, using the following reaction system: 0.2 g N -BOC-piperidone substrate was dissolved in 0.3ml DMSO and mixed, 698ul of 4.3M isopropylamine hydrochloride, 2mg PLP, 0.5~3wt recombinant crude enzyme, supplemented the reaction system with 100mM phosphate buffer pH7.0 10~16V, adjust pH to 7.0, 30℃, 200rpm to react overnight. After reaction screening, the reaction results of strains with relatively good activity are shown in Table 7. The remaining 21 transaminase mutants except Table 7 showed no increase in catalytic activity compared to wild-type transaminase activity.
[0100] Table 7:
[0101]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com