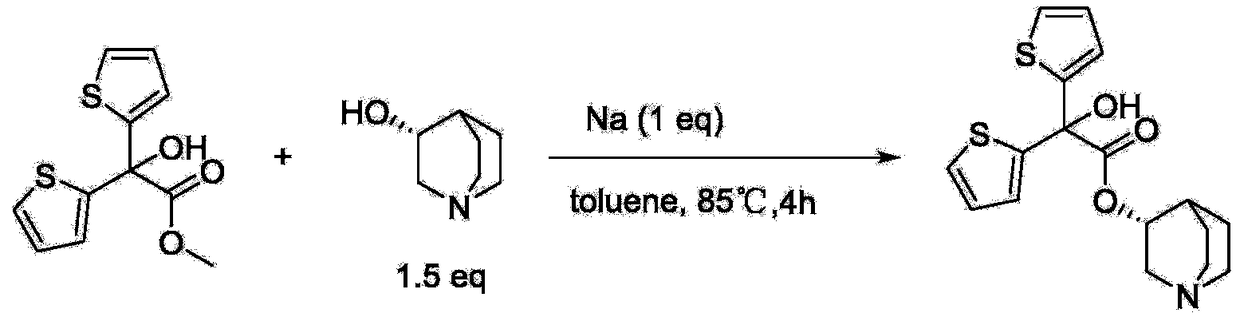
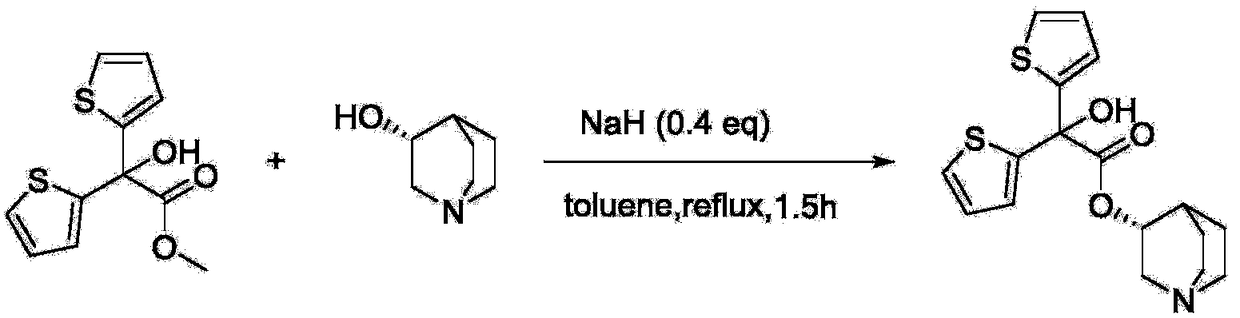
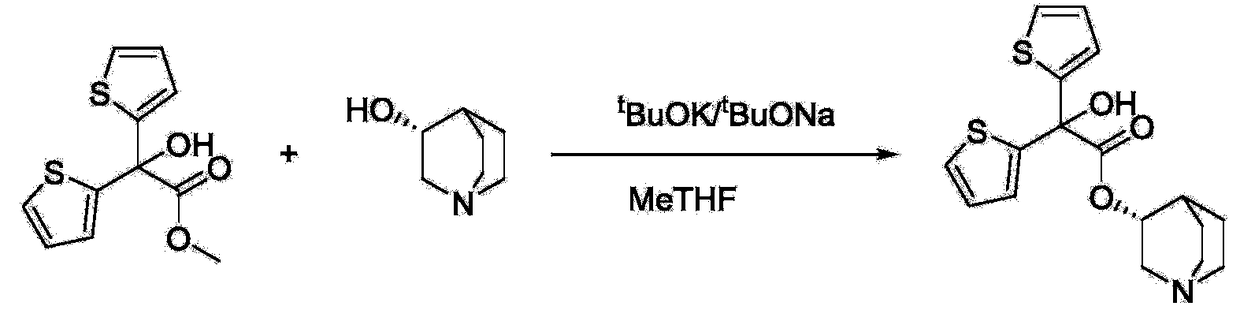
Preparation method of quinine-3-yl R-2,2-di(2-thienyl)-2-glycolate
The technology of quinine and thienyl hydroxyacetate is applied in the field of preparation of R-2,2-di-2-hydroxyacetate quinine-3-yl ester, and can solve the problem that impurity a is unfavorable for industrialized production, unsuitable for industrialized production, It is easy to generate hydrogen and other problems, and achieves the effect of being suitable for industrial production, precise and easy operation, and improved yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add methyl 2,2-dithienylglycolate (12.19g, 48mmol), R-(-)-3-quinine alcohol (5.08g, 40mmol) and sodium methoxide (1.08g, 20mmol) into a 500mL three-necked flask , then add 200ml of cyclohexane to dissolve, heat at 85°C for 1 hour, during which methanol is continuously separated, and cyclohexane is added at the same time. After the reaction, the reaction solution is poured into 100ml of dilute hydrochloric acid with a concentration of 2mol / L, and stirred After 10 minutes, the water layer was separated, and the pH of the water layer solution was adjusted to alkaline with potassium carbonate solution, filtered, and dried under reduced pressure at 50°C to obtain 11.20 g of white solid R-2,2-bis(2- Thienyl)-2-hydroxyacetic acid quinine-3-yl ester, the yield was 76.71%.
Embodiment 2
[0027] Add methyl 2,2-dithienylglycolate (12.19g, 48mmol), R-(-)-3-quinine alcohol (5.08g, 40mmol) and sodium methoxide (2.16g, 40mmol) into a 500mL three-necked flask , then add 200ml of cyclohexane to dissolve, heat at 95°C for 2 hours, during which methanol is continuously separated, and cyclohexane is added at the same time. After the reaction, the reaction solution is poured into 100ml of dilute acetic acid with a concentration of 2mol / L, and stirred After 10 minutes, separate the water layer, adjust the pH of the water layer solution to alkaline with potassium bicarbonate solution, filter and dry under reduced pressure to obtain 10.33 g of white solid R-2,2-bis(2-thienyl)- The yield of quinine-3-yl 2-hydroxyacetate is 70.75%.
Embodiment 3
[0029] Add methyl 2,2-dithienylglycolate (12.19g, 48mmol), R-(-)-3-quinine alcohol (5.08g, 40mmol) and sodium methoxide (3.24g, 60mmol) into a 500mL three-necked flask , then add 160ml of cyclohexane and 40ml of 2-methyltetrahydrofuran to dissolve, heat at 90°C for 3 hours, during which methanol is continuously separated, and at the same time, add the mixed solvent of the above ratio. After the reaction, pour the reaction solution into 100ml, the concentration is In 2mol / L dilute acetic acid, after stirring for 10min, separate the water layer, then adjust the pH of the water layer solution to alkaline with sodium carbonate solution, filter and dry under reduced pressure to obtain 11.77g of white solid R-2,2- Bis(2-thienyl)-2-hydroxyacetic acid quinine-3-yl ester, the yield is 80.50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com