A kind of human single-chain antibody and its application
A single-source antibody technology, applied in human single-chain antibody and its application field, can solve the problems of narrow action range and instability, and achieve the effects of small molecular weight, protection of tissue damage, and promotion of angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
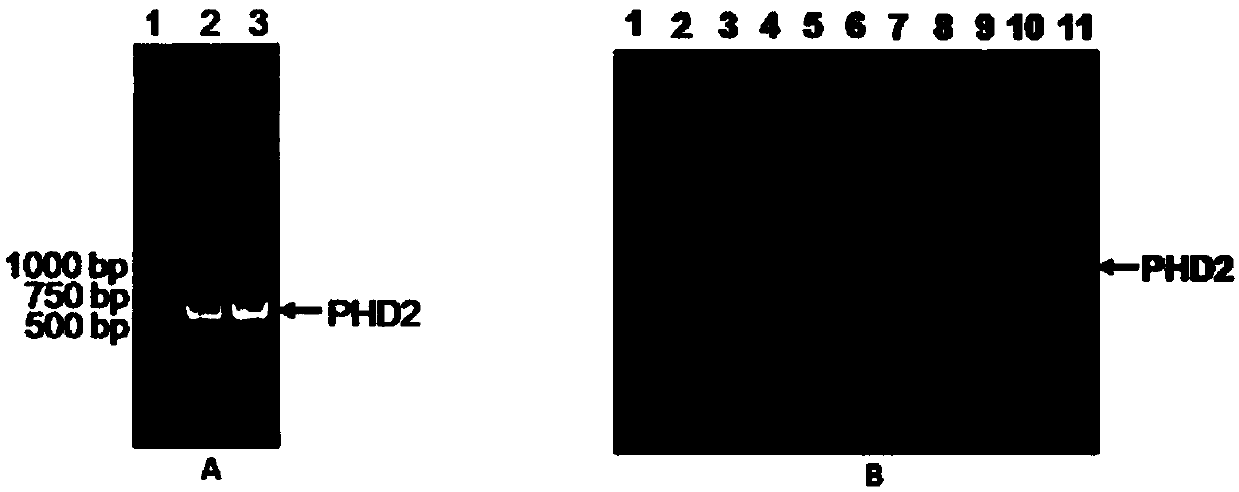
[0048] Embodiment 1: Preparation of recombinant human PHD2 protein
[0049]1. Primers required for PCR
[0050] The PHD2 primers for PCR amplification were synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the sequence is as follows:
[0051] Forward primer HumanPHD2FP is 5'-CCGGAATTCATGCTGGCGCTCGAGTACATC-3'
[0052] The reverse primer HumanPHD2RP is 5'-CCCAAGCTTCTATACTTTAGCTCGTGCTCT-3'
[0053] 2. Experimental method
[0054] (1) Total RNA was extracted from MCF-7 cells, and cDNA was obtained by reverse transcription
[0055] MCF-7 cells were cultured in a 6-well plate until the confluence reached 90-100%, the cells were collected, washed twice with pre-cooled PBS, and 0.5ml Trizol extraction solution was added to extract the total RNA of the cells. Using the extracted total RNA as the starting material, according to the instructions of the RT-PCR kit, add various components required for the reverse transcription reaction to carry out the RT ...
Embodiment 2
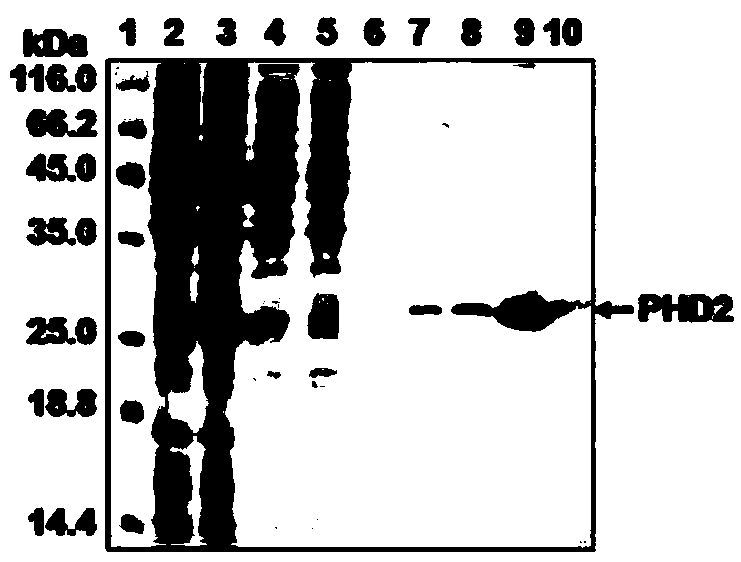
[0072] Example 2: Preparation of specific anti-PHD2 human single-chain antibody
[0073] The following "specific anti-human PHD2 human single-chain antibody" is referred to as "INP"
[0074] 1. Experimental materials
[0075] Escherichia coli (Escherichia coli) JM109, HB2151 and XL1-Blue were purchased from Beijing Dingguo Biotechnology Co., Ltd.; pUC119 plasmid was purchased from Dalian Bao Biological Engineering Co., Ltd.; HRP / Anti-M13 monoclonal antibody was purchased from Pharmacia.
[0076] 2. Test method
[0077] The acquisition of the phage human single-chain antibody library is carried out with specific reference to the methods of the literature Sblattero D et al. Nat Biotechnol, 2000, 18:74-80 and Sblattero D et al. , the final storage capacity is 1×10 11 phage human single chain antibody library.
[0078] The screening of the antibody library adopts the solid-phase antigen immunoadsorption screening method. The human PHD2 protein is diluted to 100 μg / ml with 0.05...
Embodiment 3
[0087] Example 3: Characterization of the activity of the anti-PHD2 human single-chain antibody INP
[0088] 1. Experimental materials
[0089] Reagents and materials refer to Example 1 and Example 2.
[0090] 2. Experimental method
[0091]1. ELISA analysis of the binding activity of purified anti-PHD2 single-chain antibody INP to recombinant human PHD2.
[0092] 2. Determination of INP affinity of anti-PHD2 single-chain antibody by non-competitive ELISA.
[0093] 3. Competition ELISA assay was used to detect the binding specificity of anti-PHD2 single-chain antibody to PHD2.
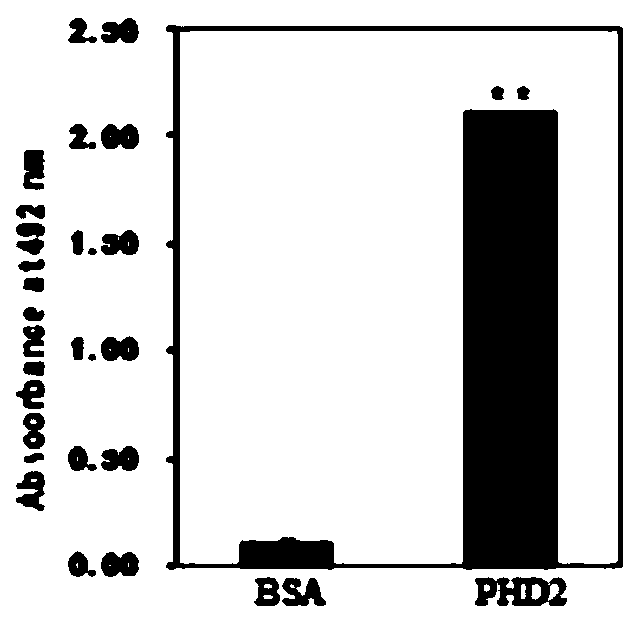
[0094] 3. Results and Analysis
[0095] 1. ELISA detection of binding activity of INP and purified PHD2 protein
[0096] In order to identify the binding activity between INP and recombinant human PHD2 protein, the purified recombinant human PHD2 protein and BSA were respectively coated at a concentration of 10 μg / ml. Anti-PHD2 mouse monoclonal antibody was used as a positive control, and INP and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com