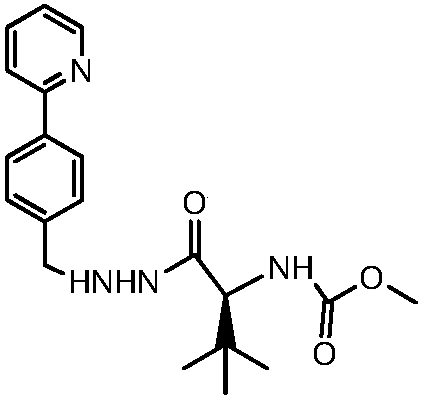
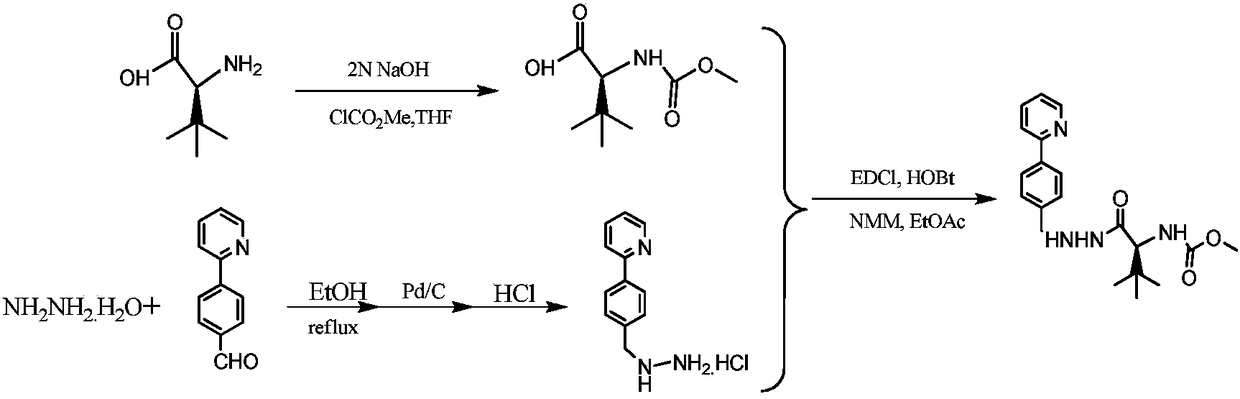
Synthetic method of atazanavir intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of low yield, high toxicity, and high cost, and achieve the effects of high yield, low cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: step (1) amide reaction:
[0023] In a 1-liter three-neck flask, add 52.4 grams of L-tert-leucine and 360 milliliters of water, add 64 grams of sodium hydroxide while stirring, cool down, slowly add 75.6 grams of methyl chloroformate, and control the temperature T≤25 °C , after dropping, heat up to 60°C, react for 18 hours, cool to room temperature, adjust PH=2 with concentrated hydrochloric acid, extract 3 times with ethyl acetate, dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and concentrate to dryness Finally, beating with 200 ml of petroleum ether; suction filtration and drying to obtain 67.6 g of white solid. Yield: 89%.
Embodiment 2
[0024] Embodiment 2: step (2) condensation and hydrogenation reaction:
[0025] In a 250 ml three-necked flask, add 18.3 grams of 4-(2-pyridyl)-benzaldehyde, 5.88 grams of 85% hydrazine hydrate, and 150 milliliters of ethanol, heat the oil bath to 80°C, react for 2-3 hours, and cool down to T= At 25°C, add 1.4 g of palladium carbon with nitrogen gas, and then change to hydrogen gas, react for 24 hours, filter, recover palladium carbon, pass hydrogen chloride to the filtrate in an ice bath, until PH = 2-3, filter with suction, and dry to obtain light yellow Solid 23.6 grams. The yield of this step is 90%.
Embodiment 3
[0026] Embodiment 3: step (3) condensation reaction:
[0027] In a 500 ml three-necked flask, add 16.06 grams of N-methoxycarbonyl-L-tert-leucine, 16.26 grams of EDCl, 11.46 grams of HOBt, 240 milliliters of ethyl acetate, and add 9.36 grams of N-methylmorpholine while stirring After 30 minutes of reaction, 20.8 grams of 2-[4-(2-pyridyl)-benzyl]hydrazine hydrochloride, 9.36 grams of triethylamine, and 160 milliliters of ethyl acetate were added respectively, and the reaction was carried out for 4 hours. Sodium, water, and saturated brine were washed, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 28.5 grams of a crude light yellow solid, which was recrystallized with ethyl acetate to obtain 21 g of a white solid with a purity of more than 99%. That is the intermediate of atazanavir. The yield of this step is 80%.
[0028] The overall yield of the entire method scheme was 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com