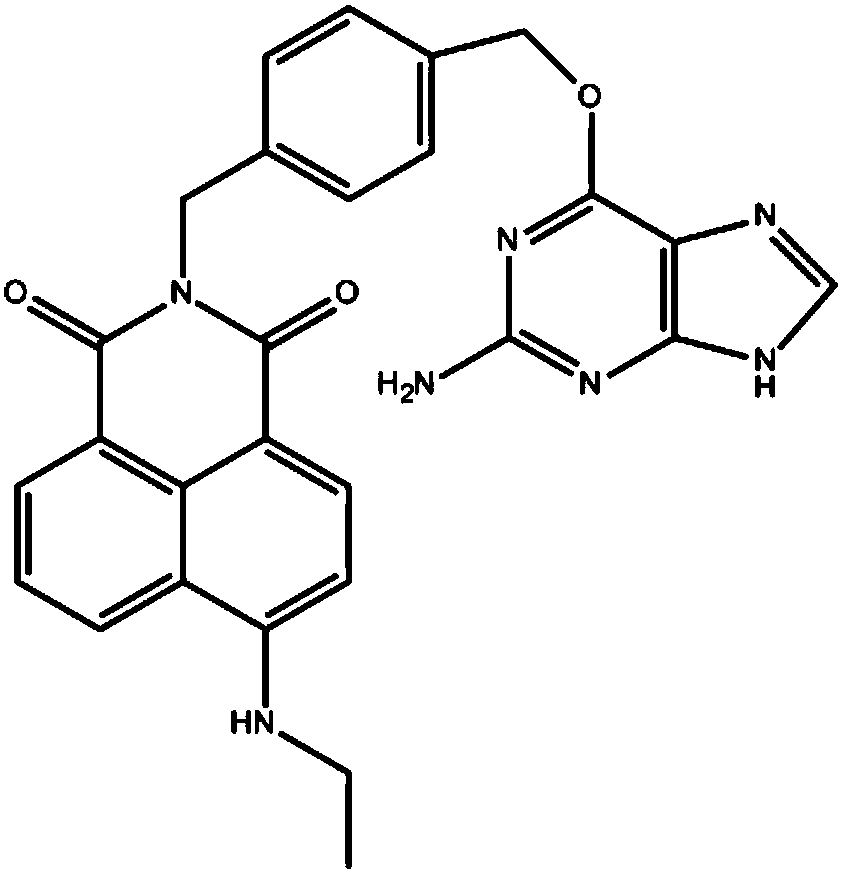
Small-molecular fluorescent probe for SNAP protein labeling as well as synthesis method and application of small-molecular fluorescent probe
A technology of fluorescent probe and synthesis method, applied in the field of biological analysis and detection, can solve the problems of single fluorescence spectrum, slow response, low sensitivity, etc., and achieve the effects of simple synthesis route, mild reaction conditions, and simple and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
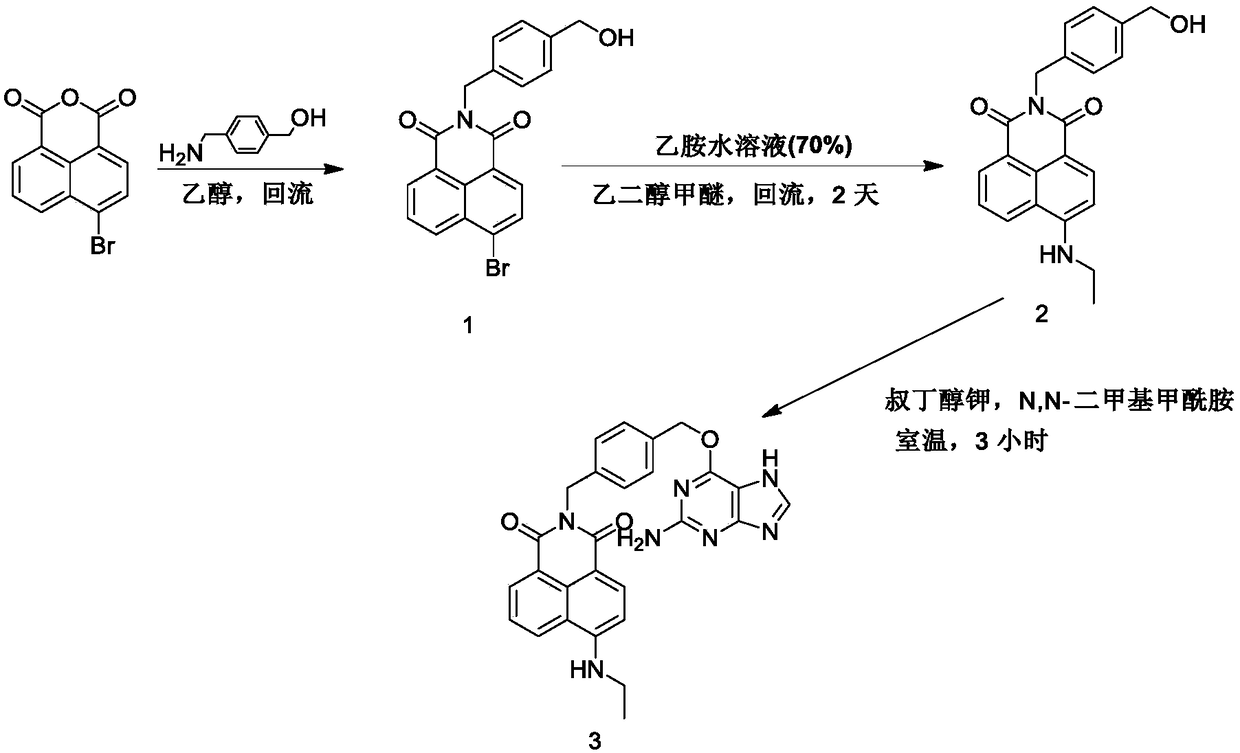
[0039] Example 1: Synthesis of small molecule fluorescent probes for protein labeling
[0040] (1) Synthesis of intermediates:
[0041]
[0042] 4-Bromo-1,8-naphthalene anhydride (2.77 g, 10 mmol) was dissolved in 25 mL of absolute ethanol, and 4-aminomethylbenzyl alcohol (1.37 g, 10 mmol) was added to the reaction solution. The reaction solution was heated to reflux. After 6 hours, ethanol was removed under reduced pressure, and column chromatography (dichloromethane: methanol = 100:1) gave 3.08 g of white solid (intermediate compound 1), with a yield of 78%.
[0043]
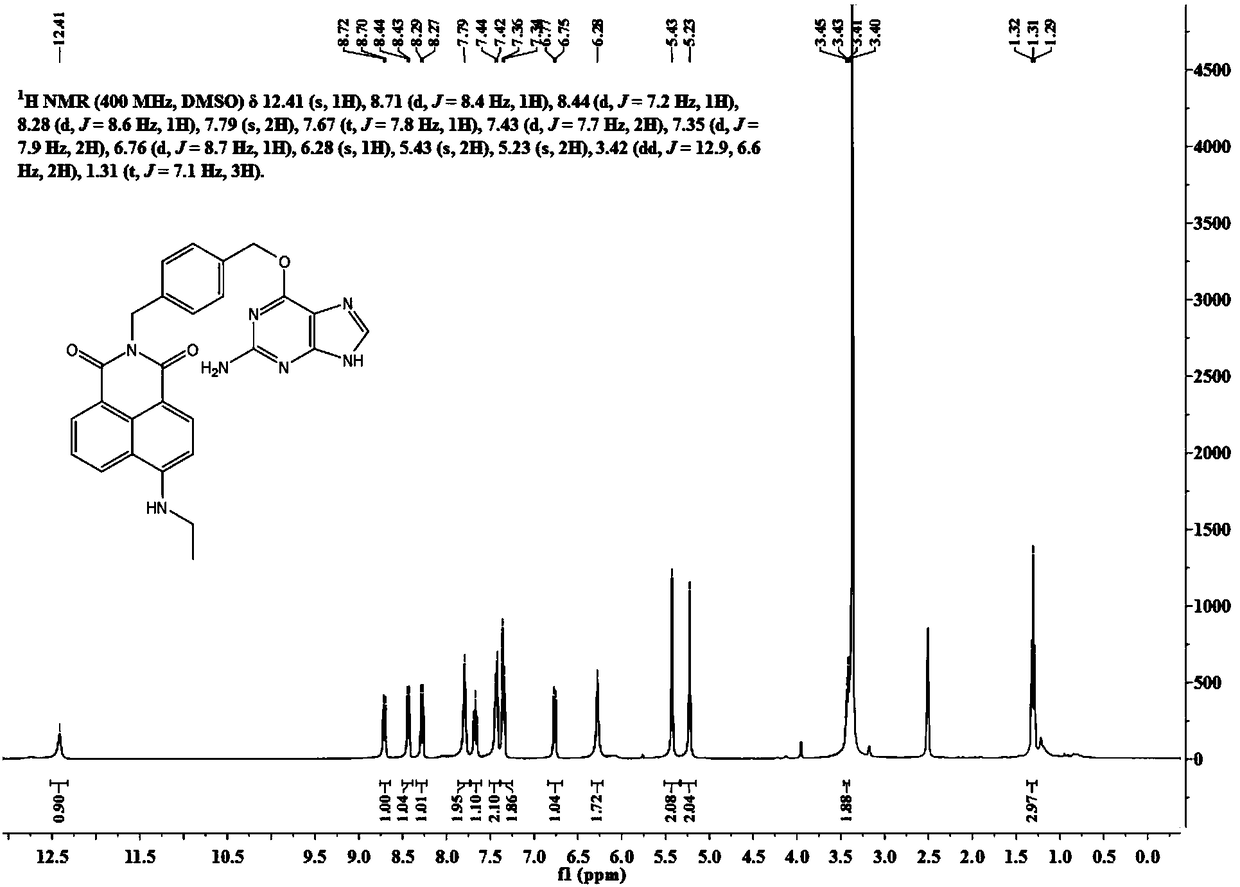
[0044] Compound 1 (200 mg, 0.51 mmol) was dissolved in 5 mL of ethylene glycol methyl ether, and 100 microliters of 70% ethylamine aqueous solution was added thereto under nitrogen protection. The reaction solution was slowly heated to 120° C. and stirred for 2 days. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichlorometh...
Embodiment 2
[0048] Example 2: Fluorescence changes after fluorescent probe reacts with SNAP
[0049] The probe was dissolved in DMSO solution to prepare 2 mM stock solution, and 1 μM probe and 5 μM SNAP were respectively prepared in 4 mL of 20 mM PBS solution with pH=7.4. A 1 μM probe was additionally prepared with 5 μM SNAP. Stir at 37 °C for 1 h. Measure the fluorescence to get Figure 5 .
[0050] Figure 5 In the presence of only the probe (1μM) showed weak fluorescence. When the probe reacts with SNAP, the fluorescence is significantly enhanced, several times stronger, and the emission wavelength is about 530nm.
Embodiment 3
[0051] Embodiment 3: the kinetics of fluorescent probe and SNAP reaction
[0052] Add 1μM probe to 4mL, 2μM SNAP solution, measure the fluorescence change at 530nm wavelength (excitation wavelength is 460nm) to get Image 6 , the results showed that the fluorescence increased rapidly after adding the probe, indicating that the probe could specifically recognize and react rapidly with SNAP.
[0053] Fluorescence is significantly increased by about 30 times, through which the interference of background light can be excluded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com