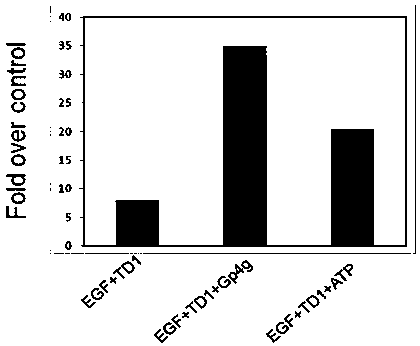Percutaneous absorption promotion composition and application thereof
A composition and transdermal technology, which can be used in drug combinations, inactive components of polymer compounds, skin diseases, etc., can solve the problems of high-energy phosphate bonds easily broken, ATP instability, etc., to improve transdermal transport efficiency and chemical properties. Stable and low cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 The effect of the combination of Gp4g and TD-1, and the combination of ATP and TD-1 on the transdermal efficiency of EGF in the abdominal skin tissue of rats.
[0038] TD-1 is a short peptide of ACSSSPSKHCG; EGF is a recombinant human epidermal growth factor (ATP helps TD-1 mediate the fusion transdermal protein drug transdermal in this application, taking human epidermal growth factor TD-1-huEGF as an example, the following text huEGF is represented by EGF)
[0039] Gp4g (diguanosine tetraphosphate) was purchased from Tianjin Qiangweite Biotechnology Co., Ltd. (product number E0012), INCI name: ARTEMIA EXTRACT. Adenosine triphosphate (ATP) was purchased from Bio Basic Inc., Canada (Product No. AB0020). Recombinant human epidermal growth factor (EGF) was purchased from Shenzhen Huashengyuan Gene Engineering Development Co., Ltd.
[0040] After abdominal depilation of SD rats (Shanghai Slack Experimental Animal Co., Ltd., all rats are from this source), intac...
Embodiment 2
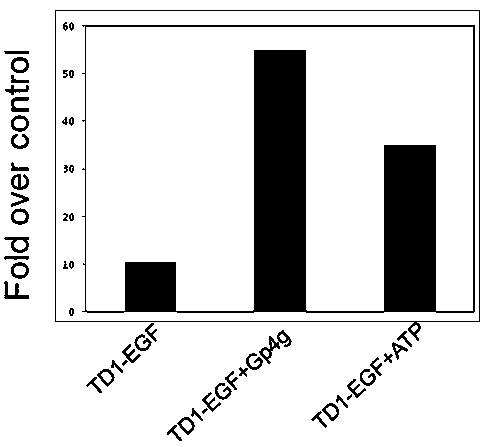
[0051] Example 2 Comparison of TD-1-EGF and TD-1-EGF+ATP Transdermal Drugs
[0052] TD-1-EGF comes from Fujian Longsheng Biotechnology Co., Ltd., product number LS-102. The recombinant plasmid was constructed by Fujian Longsheng Biotechnology Co., Ltd., and the target protein was expressed and purified using the E. coli prokaryotic expression system.
[0053] TD-1-EGF is a proven transdermal fusion protein drug (Ruan R Q, Wang S S, WangCL, et al. Transdermal delivery of human epidermal growth factor facilitated by a peptide chaperon.[J]. European Journal of Medicinal Chemistry, 2013, 62(62C):405-409.), in this example, during the transdermal administration of TD-1-mediated transdermal fusion protein drug TD-1-EGF, exogenously supplement ATP to skin cells A precursor, Gp4g (diguanosine tetraphosphate), was synthesized to study the effect of Gp4g on the transdermal efficiency of the transdermal fusion protein drug TD-1-EGF.
[0054] After abdominal depilation of SD rats (Shang...
Embodiment 3
[0073] Example 3 A skin external preparation containing a transdermal administration composition, which includes the following components in mass percent:
[0074] TD-1-EGF (transdermal EGF, specification 20ug / mL): 1%
[0075] Gp4g: 1%
[0076] Sodium hyaluronate: 0.05%
[0077] Aloe Vera Extract: 0.5%
[0078] Centella asiatica extract: 0.05%
[0079] Rosemary Leaf Extract: 0.05%
[0080] Glycyrrhiza glabra extract: 0.05%
[0081] VC polysaccharide: 0.5%
[0082] Butanediol: 4%
[0083] Hydroxyethylurea: 0.5%
[0084] Glyceryl polyethers-26: 2%
[0085]Preservatives (A631): 0.5%;
[0086] Flavor: 0.02%;
[0087] The above ingredients were dissolved in purified water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com