Preparation method of canrenone intermediate
An intermediate, canrenone technology, which is applied in the field of preparation of canrenone intermediates, can solve problems affecting production efficiency, quality and yield, and achieve the effects of low production cost, less environmental pollution, and increased concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
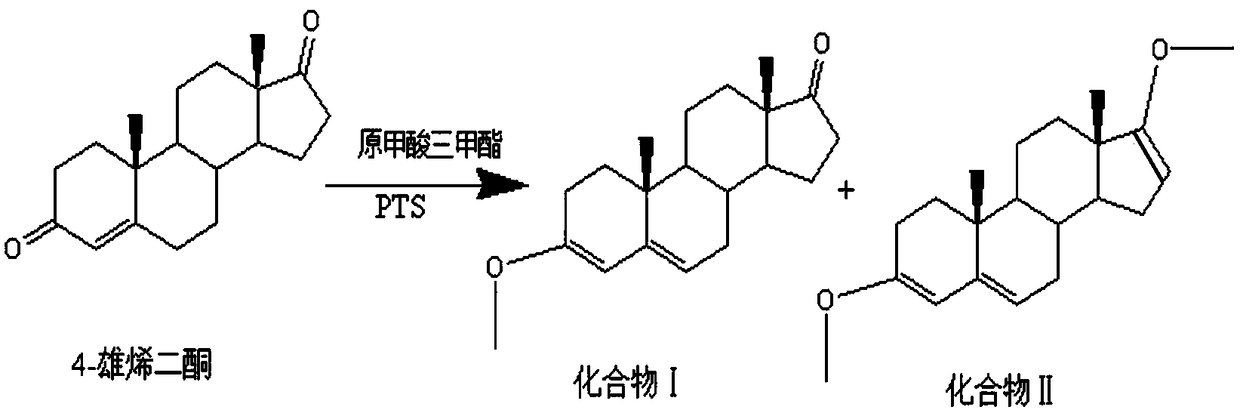
[0027] Such as figure 1 The method for preparing a canrenone intermediate includes the following steps: adding 30 g of 4-androstenedione, 120 ml of tetrahydrofuran, and 0.9 g of p-toluenesulfonic acid to a three-necked flask under nitrogen protection, and heating to 30°C, Slowly add 60ml of trimethyl orthoformate, stir at 38°C for 3h, cool to -5°C, add 15ml of water at -5°C, hydrolyze for 6min, immediately add 30ml of 2% sodium carbonate solution to stop the reaction, and pour into the reaction solution Put into 240ml ice water, stir for 1h, filter with suction, wash with a small amount of water, and dry.
[0028] 30.84 g of the product (Compound I) was obtained, with a yield of 102.8% and a purity of 99.5%.
Embodiment 2
[0030] Such as figure 1 The method for preparing a canrenone intermediate includes the following steps: adding 50 g of 4-androstenedione, 200 ml of tetrahydrofuran, and 2.5 g of p-toluenesulfonic acid into a three-necked flask under the protection of nitrogen, and heating to 35°C, Slowly add 200ml of trimethyl orthoformate, stir at 45°C for 4h, cool to -10°C, add 50ml of water and stir for 10min, immediately add 50ml of 5% sodium carbonate solution to stop the reaction, pour the reaction solution into 400ml of ice water and stir for 1h. Filter by suction, wash with a little water, and dry.
[0031] The product (Compound I) 51g was obtained with a yield of 101% and a purity of 99.2%.
Embodiment 3
[0033] Such as figure 1 The method for preparing a canrenone intermediate includes the following steps: under the protection of nitrogen, add 100g of 4-androstenedione, 400ml of tetrahydrofuran, and 1g of p-toluenesulfonic acid into a three-necked flask, and raise the temperature to 30°C slowly. Add 400ml of trimethyl orthoformate, stir at 30°C for 2h, cool to 10°C, add 100ml of water at 10°C and stir for 3min, immediately add 100ml of 1% sodium carbonate solution to stop the reaction, pour the reaction solution into 800ml of ice water and stir for 1h , Suction filtration, washing with a little water, and drying.
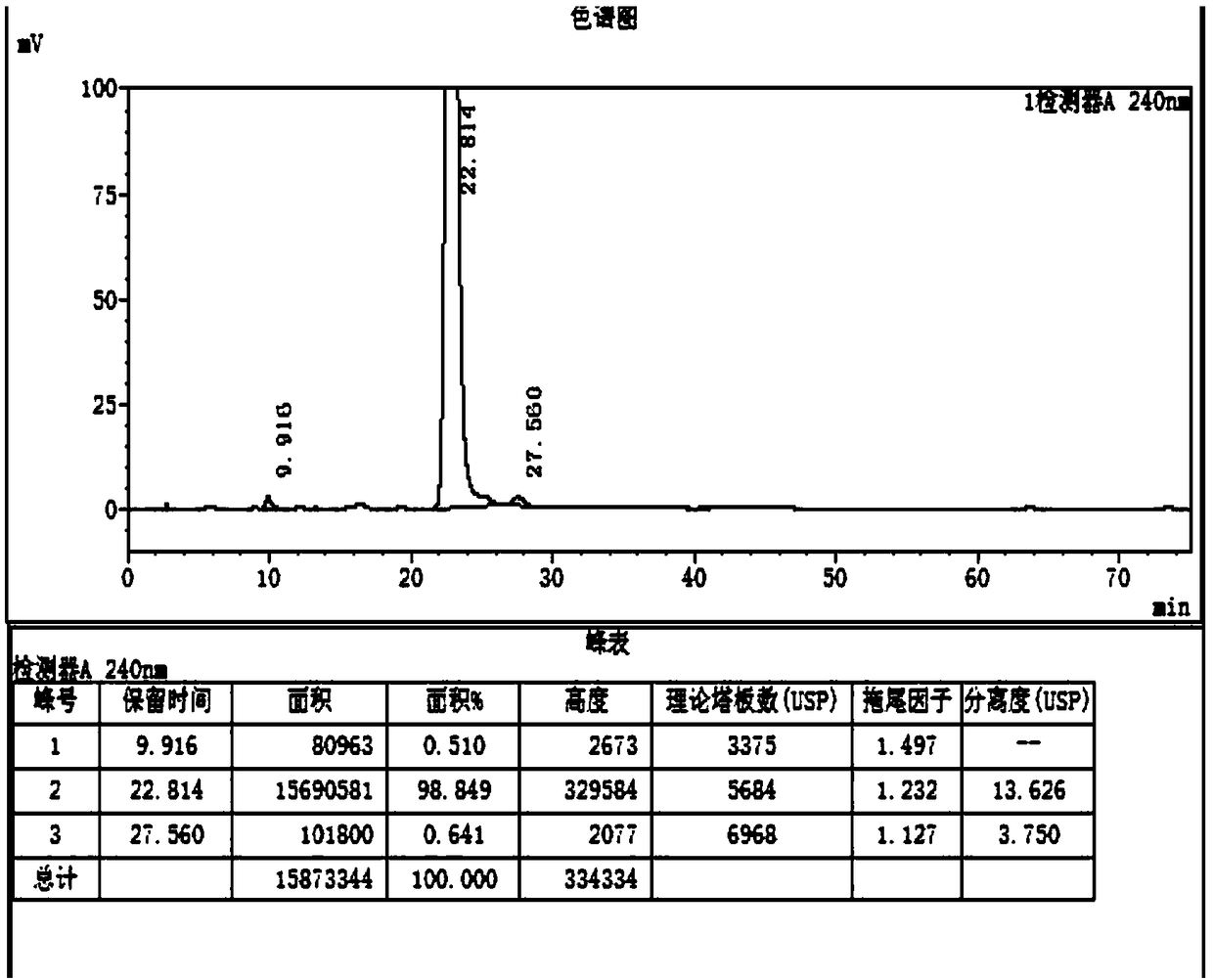
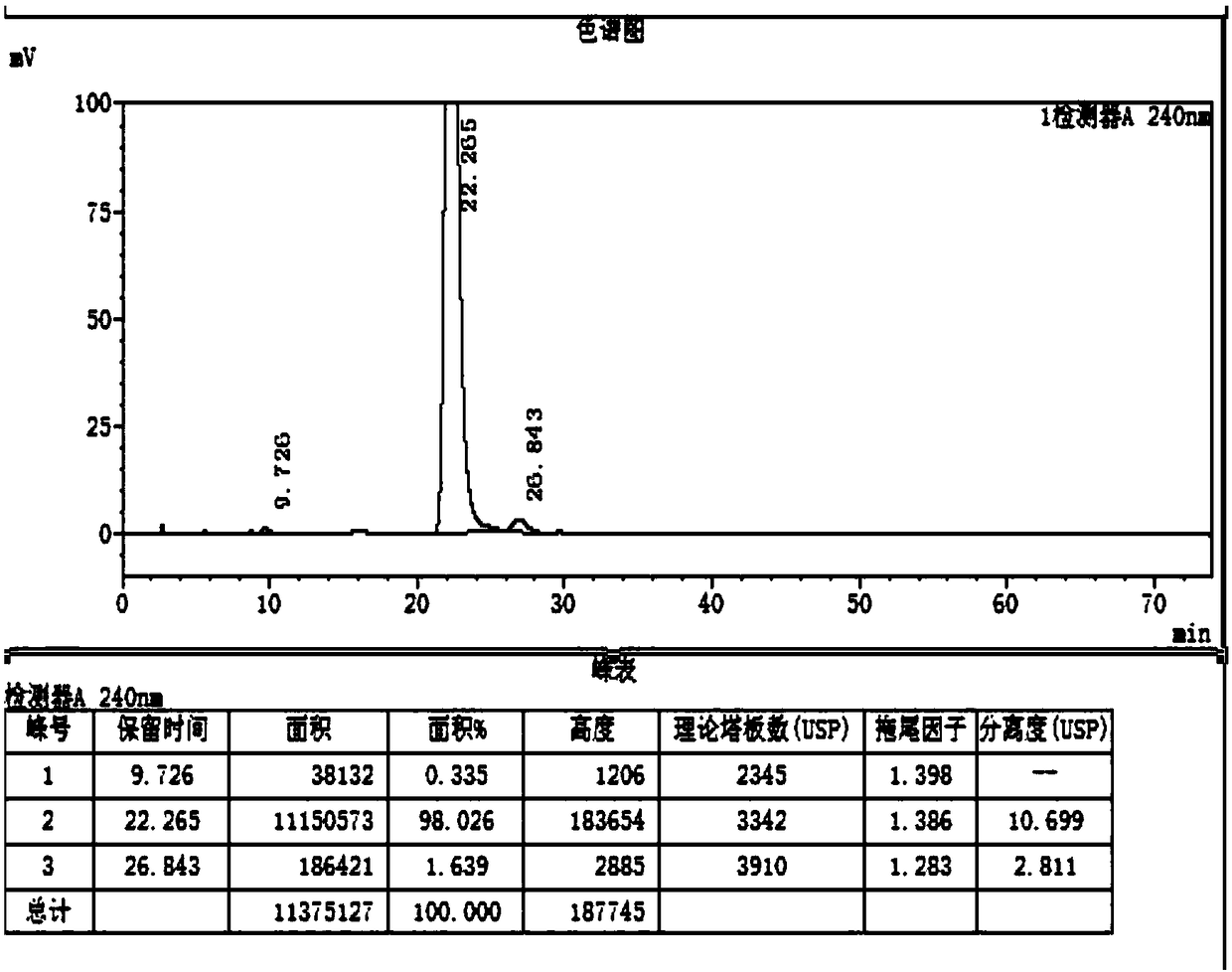
[0034] Obtain 100g of the product (Compound I) with a yield of 100%, such as figure 2 Said, the purity of the prepared compound I is 98.8%, as image 3 The purity of the standard sample is 98.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com