Acrylate resin containing hyperbranched structure and preparation method thereof
An acrylate, branched structure technology, applied in polyester coatings, coatings, etc., can solve the problems of high cost and long reaction time, and achieve the effect of low viscosity characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment one: the synthesis of polyester type hyperbranched monomer
[0043]Example 1 Using xylene as a solvent, add 92 grams of glycerin to the flask, add 296 grams of phthalic anhydride in batches at 100°C for 0.5 hours to make it react slowly, gradually raise the temperature to 140°C, keep at reflux for 10 minutes and then cool down. 98 grams of maleic anhydride was added at 100°C, and the temperature was gradually raised to reflux and kept for 10 minutes. Cool down and add 402 grams of trimethylolpropane, raise the temperature to reflux state, keep the esterification until the acid value is below 5 mgKOH / g, then add 244 grams of benzoic acid and 200 grams of lauric acid, and continue to reflux to esterify to the acid value below 5 mgKOH / g.
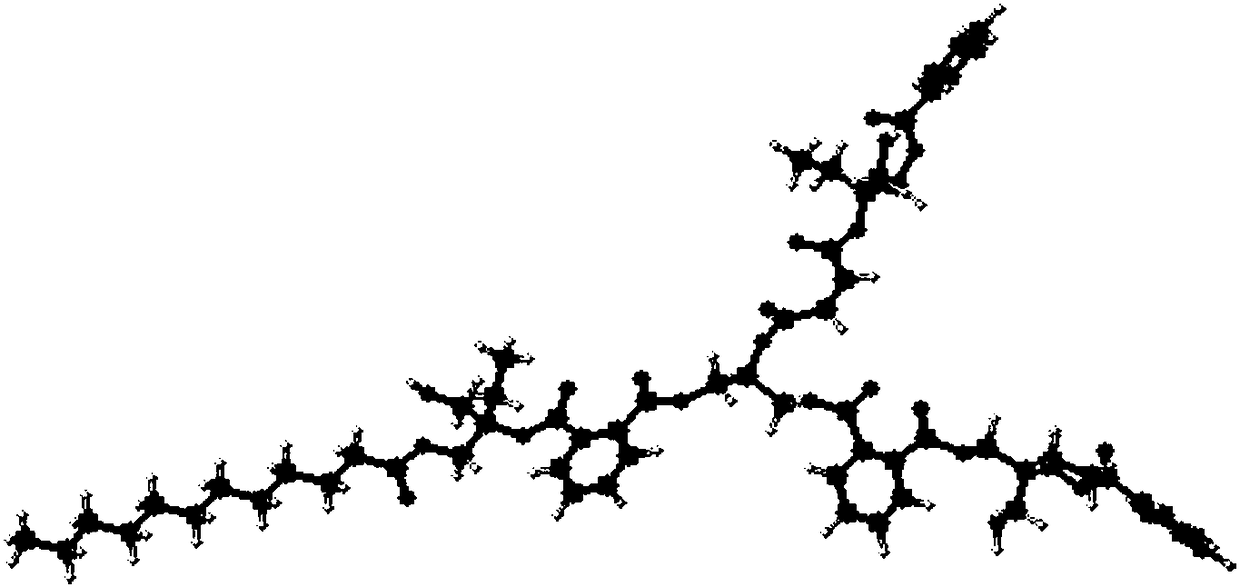
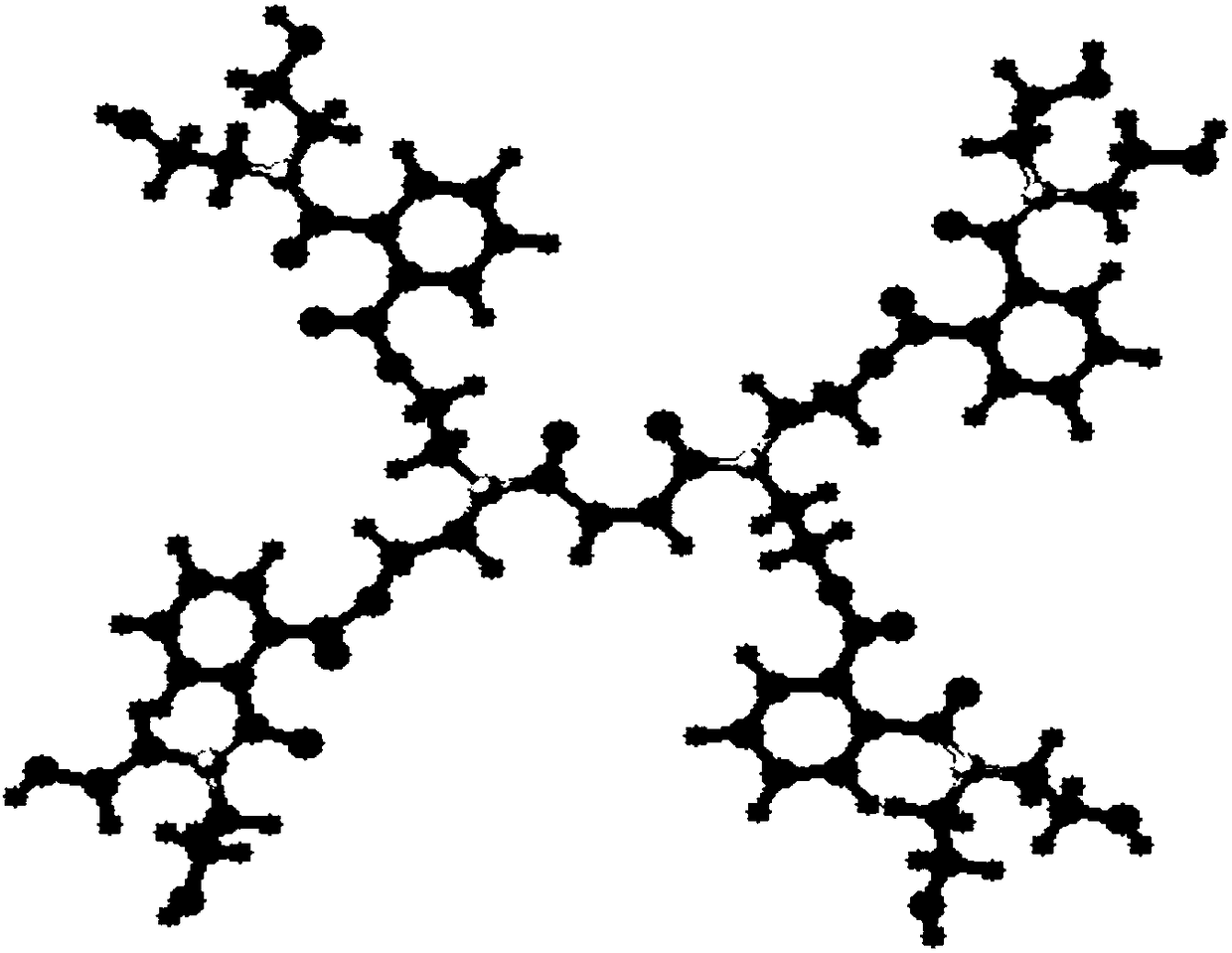
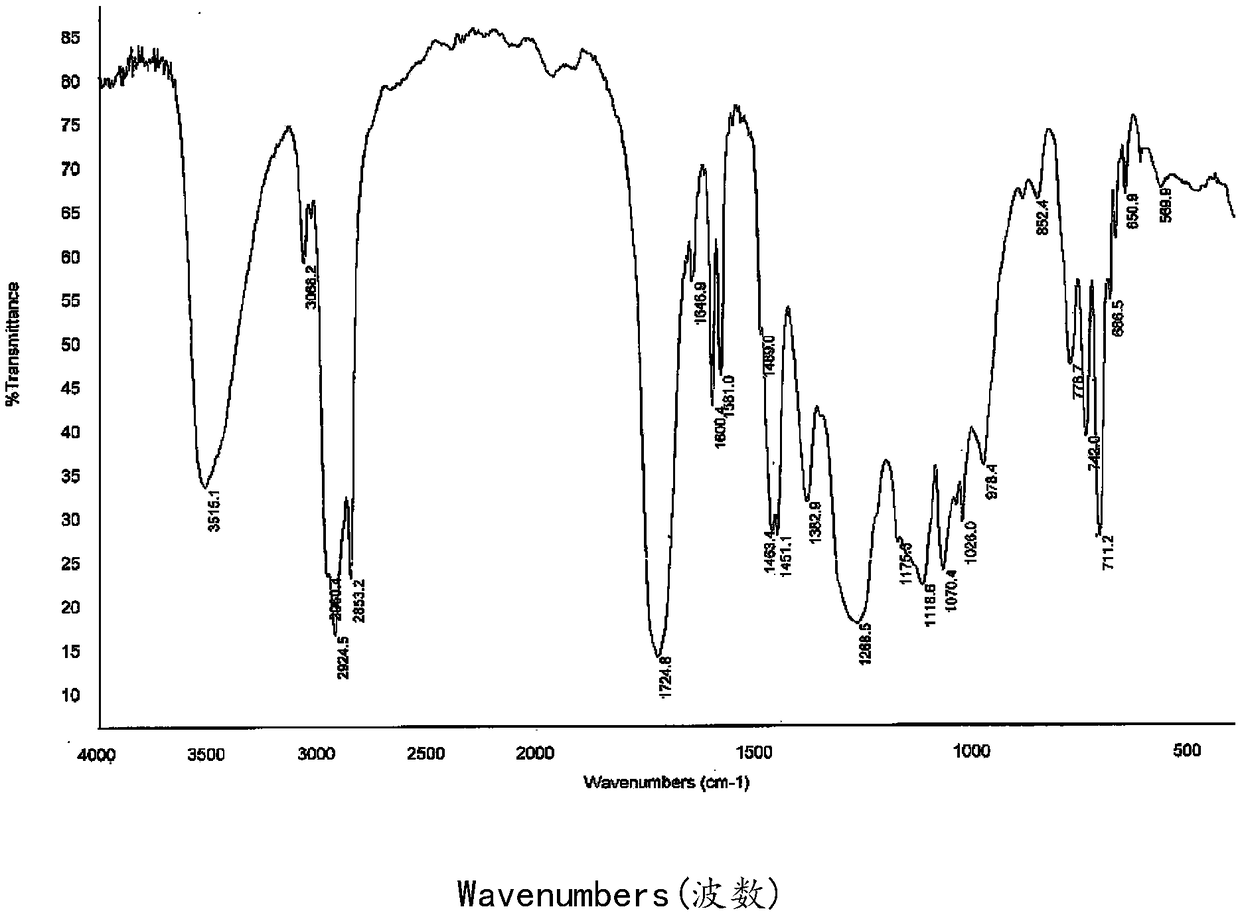
[0044] see figure 1 , is the molecular structure diagram of the polyester type hyperbranched monomer of the present embodiment, and the hydroxyl value and acid value analysis of the intermediate product in the synthesis proce...
Embodiment 2
[0051] Embodiment two: the synthesis of polyester type hyperbranched monomer
[0052] Using xylene as a solvent, add 92 grams of glycerin to the flask, add 292 grams of adipic acid in batches at 100°C for 0.5 hours to make it react slowly, gradually raise the temperature to 140-240°C and reflux until the acid value reaches the specified value, cool down, 100 98 g of maleic anhydride was added at ℃, and the temperature was gradually raised to reflux state and kept for 10 minutes. Cool down and add 402 grams of trimethylolpropane, raise the temperature to reflux state, keep the esterification until the acid value is below 5 mgKOH / g, then add 244 grams of benzoic acid and 200 grams of lauric acid, and continue to reflux to esterify to the acid value below 5 mgKOH / g.
Embodiment 3
[0053] Embodiment three: the synthesis of polyester type hyperbranched monomer
[0054] Using xylene as a solvent, add 92 grams of glycerin to the flask, add 296 grams of phthalic anhydride in batches at 100°C for 0.5 hours to make it slowly react, gradually increase the temperature at 140°C and keep it under reflux for 10 minutes, then cool down, add 98 grams at 100°C Maleic anhydride, gradually warming up to reflux state, keeping for 10 minutes. Cool down and add 402 grams of trimethylolpropane, raise the temperature to reflux state, keep the esterification until the acid value is below 5 mgKOH / g, then add 244 grams of benzoic acid and 284 grams of stearic acid, continue to reflux and esterify to the acid value below 5 mgKOH / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com