Au@Au/Pt core-shell structure nano catalyst applied to alcohol fuel cells
A nano-catalyst and fuel cell technology, applied in nanotechnology, fuel cell, nanotechnology, etc. for materials and surface science, can solve the problems of low catalytic efficiency and easy poisoning of ethanol fuel cells, and achieve good and stable catalytic activity Good performance and load reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Au@AuPt core-shell nanostructures
[0036] 1.1 Preparation of Au nanoparticles
[0037] (1) Before the experiment, prepare several 25 mL beakers, medicine spoons, a 50 mL beaker, 2 magnetic stir bars, and a 50 mL graduated cylinder, soak in aqua regia, wash and dry before use. As well as 5-50 μL pipettes, 100-1000 μL pipettes.
[0038] (2) Take a clean beaker, pour in a small amount of deionized water, put it in the freezer of the refrigerator and freeze it for later use. The medicine spoon is washed with deionized water, washed and dried with ethanol, weigh 0.364 g of cetyl trimethyl ammonium bromide with an electronic balance, and add 10 mL of deionized water with a pipette to the beaker to dissolve. Weigh 0.033 g of sodium borohydride with an electronic balance, take 10 mL of ice water, and pour it into a 25 mL beaker to dissolve. At 27°C, use a 5-50 μL pipette to pipette 42.5 μL of chloroauric acid (1%) into the prepared 5 mL of cetyltrimet...
Embodiment 2
[0051] Example 2 Preparation of core-shell structure Physical and chemical properties characterization
[0052] 2.1 Morphology
[0053] The transmission electron microscope pictures of gold nanoparticles, Au@Ag core-shell nanocubes, Au@AuPt core-shell nanostructures and Au@Pt core-shell nanoparticles in Comparative Example 1 in Example 1 are as follows figure 1 shown in (a)-(c). It can be seen from the figure that the obtained Au@AuPt core-shell nanostructures are concave cubes with an average particle size of 29±4 nm. The gold nanoparticles and Au@Ag core-shell nanocubes are monodisperse with an average particle size of 10±2 nm, and the average particle size of Au@Pt core-shell nanoparticles is 33±3 nm.
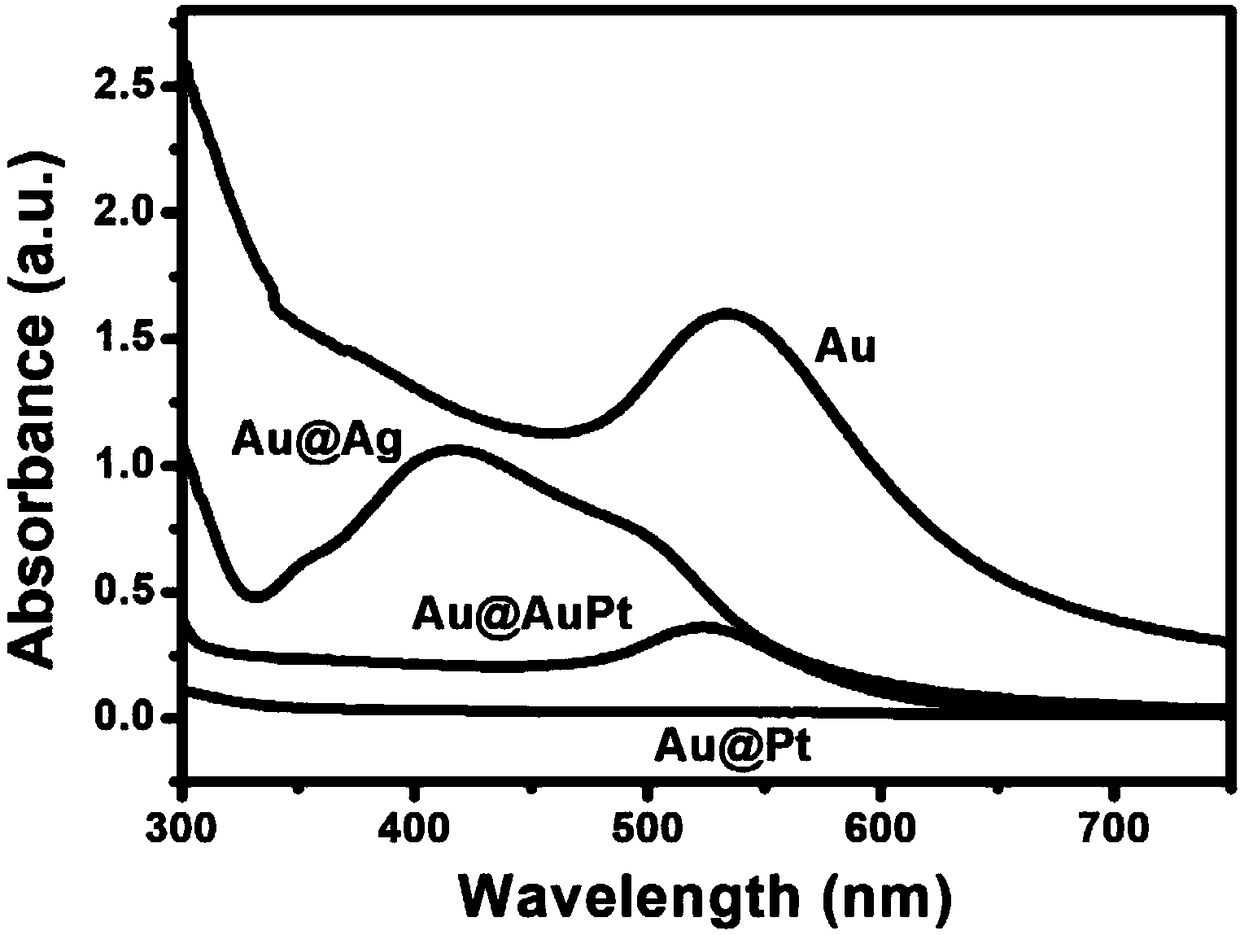
[0054] 2.2 UV-Vis Spectrum
[0055] The gold nanoparticles, Au@Ag core-shell nanocubes, Au@AuPt core-shell nanostructures in Example 1 and Au@Pt core-shell nanoparticles in Comparative Example 1 were subjected to ultraviolet-visible light spectral scanning, and the spectr...
Embodiment 3
[0056] Example 3 The catalytic effect of preparing core-shell structures on ethanol fuel cells
[0057] 3.1 Chronoamperometric and cyclic voltammetry characteristics
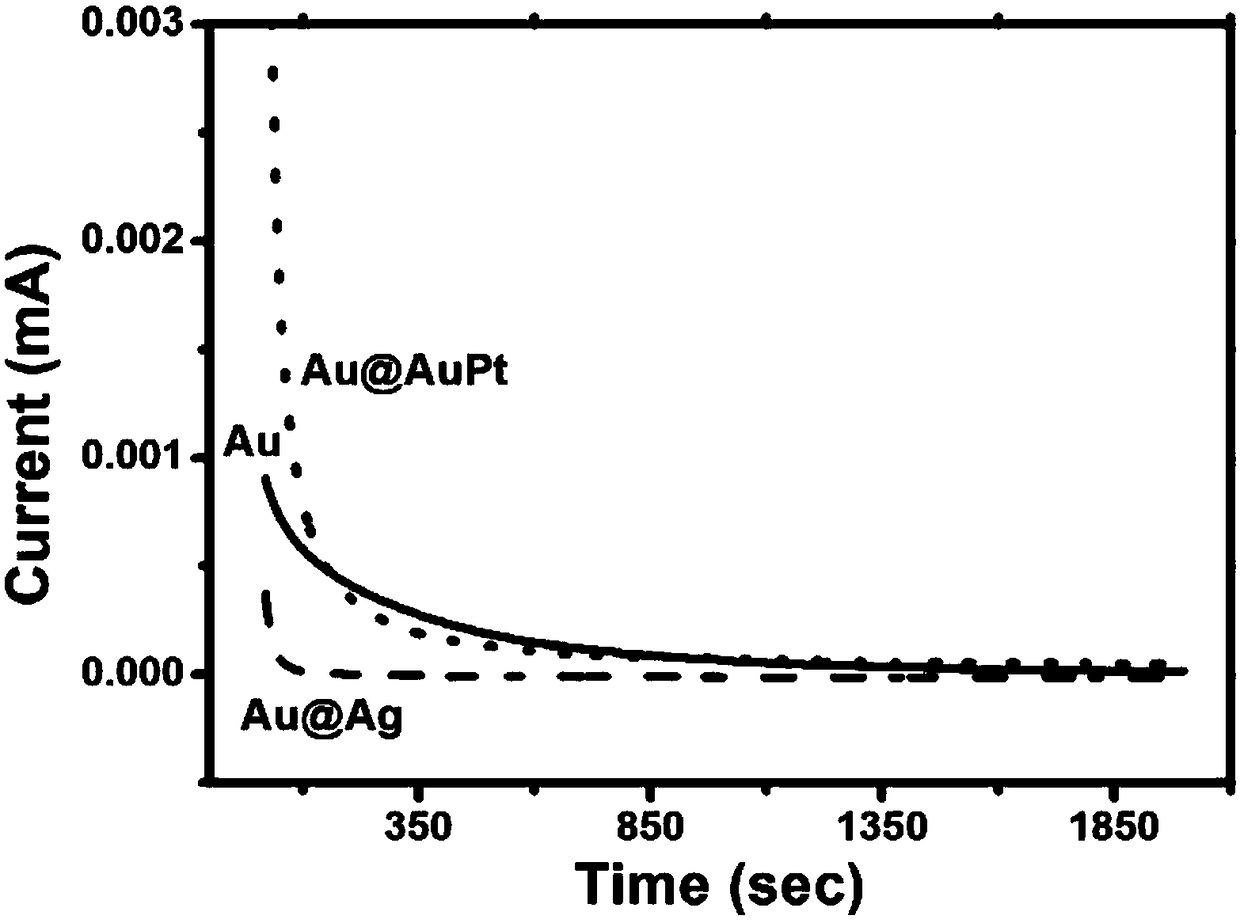
[0058] Chronoamperometric and voltammetric characteristics of Au@Ag core-shell nanocubes, Au@AuPt core-shell nanostructures and Au@Pt core-shell nanoparticles in Comparative Example 1 were tested. The detection conditions were as follows: 1.0 M sodium hydroxide and 1.0 M ethanol mixed solution were mixed, the scanning voltage was -1V~0.6V, and the scanning speed was 50 mV / s.
[0059] Chronocurrent results such as image 3 As shown, the Au@AuPt core-shell nanocubes have good catalytic performance and high stability.
[0060] The result of the voltammetric cycle curve is as follows Figure 4 Shown: the peak potential of Au@Pt core-shell nanoparticles (about -0.3 V) is lower than that of gold nanoparticles (about 0.2 V), indicating that the platinum shell enhances the catalytic effect on ethanol; Au@Au / Pt core ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com