Asymmetric hydronitrogen-pyridine-nickel metal catalyst, and preparation method and application of catalyst
A metal catalyst, asymmetric technology, applied in the field of electrochemistry, to achieve the effect of stabilizing intermediates, promoting coordination, and benefiting the electrocatalytic reduction of carbon dioxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
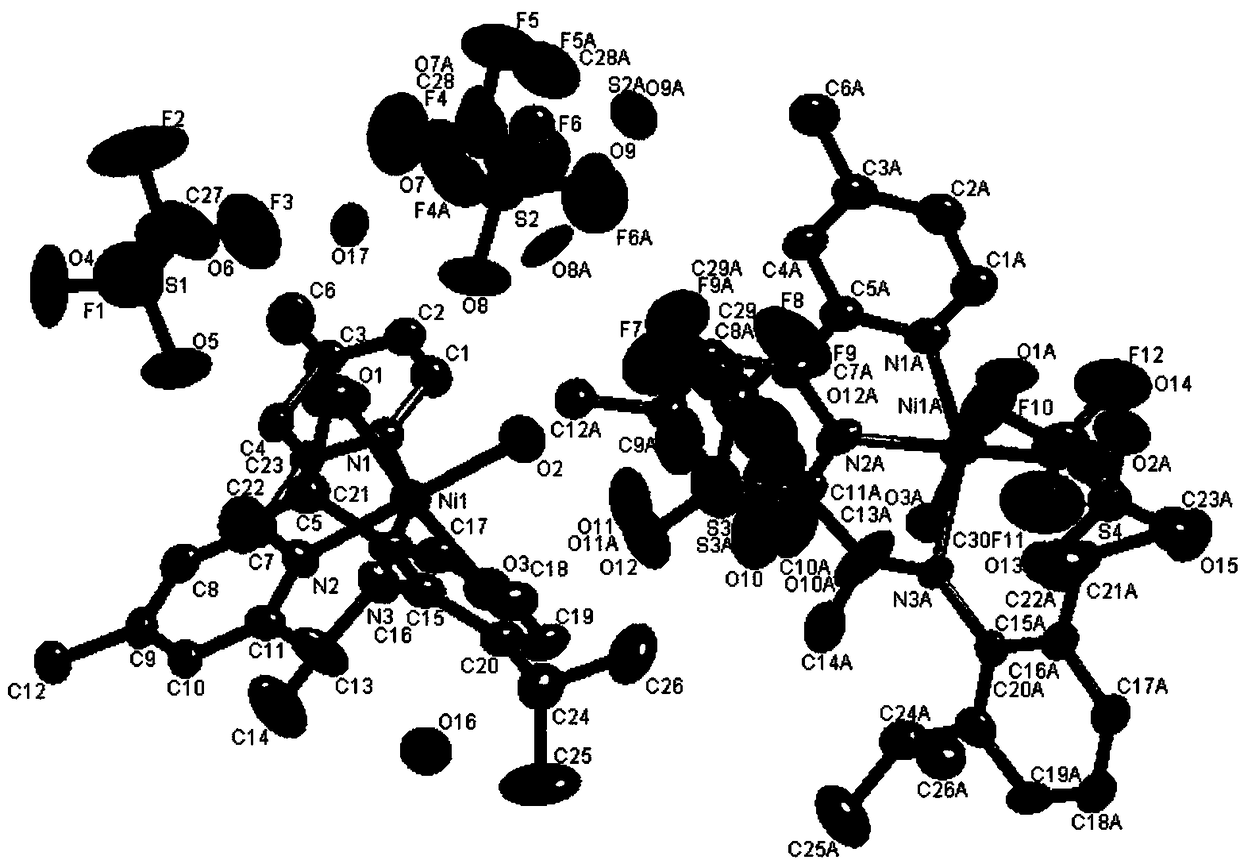
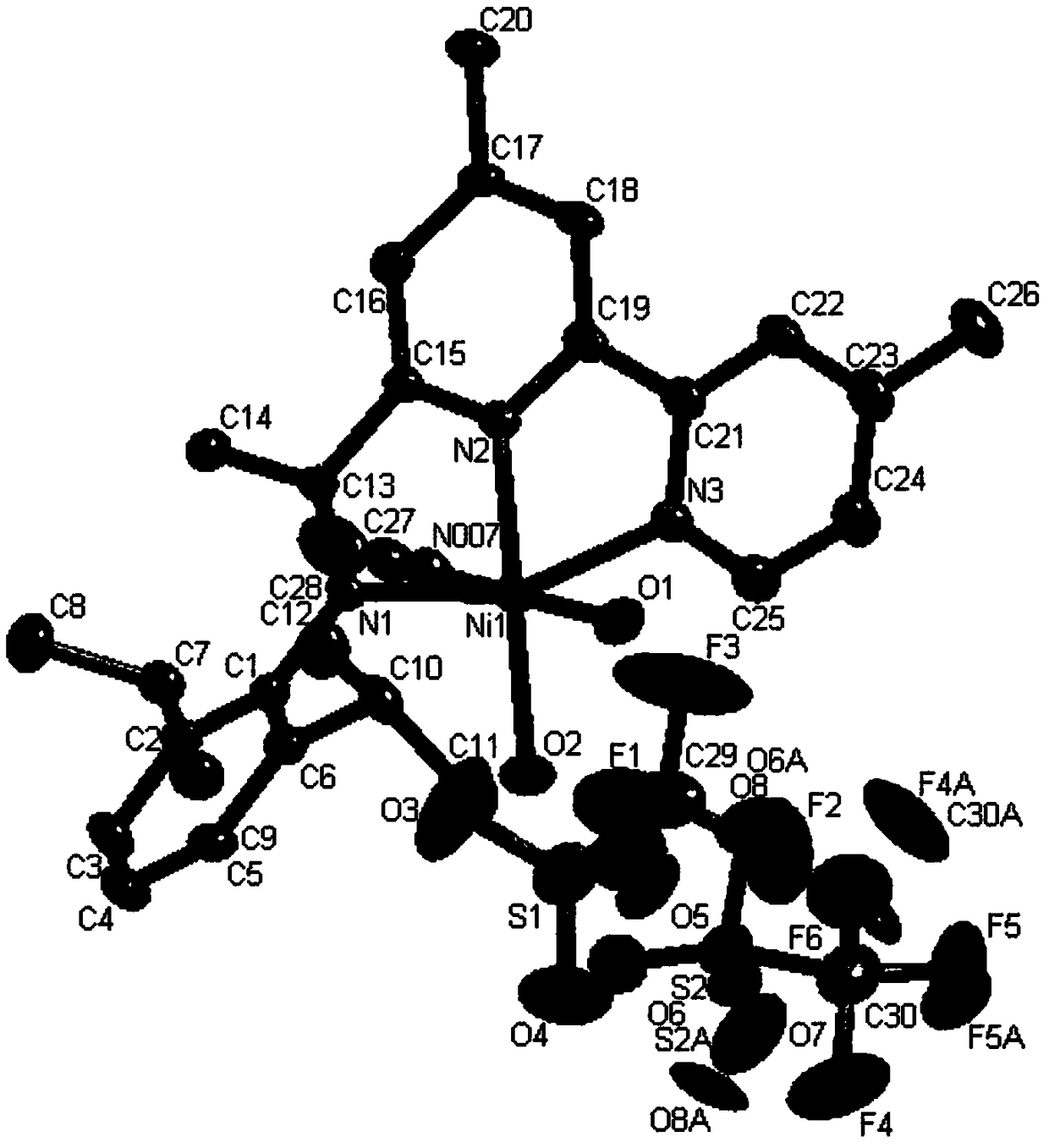
Image
Examples
Embodiment 1
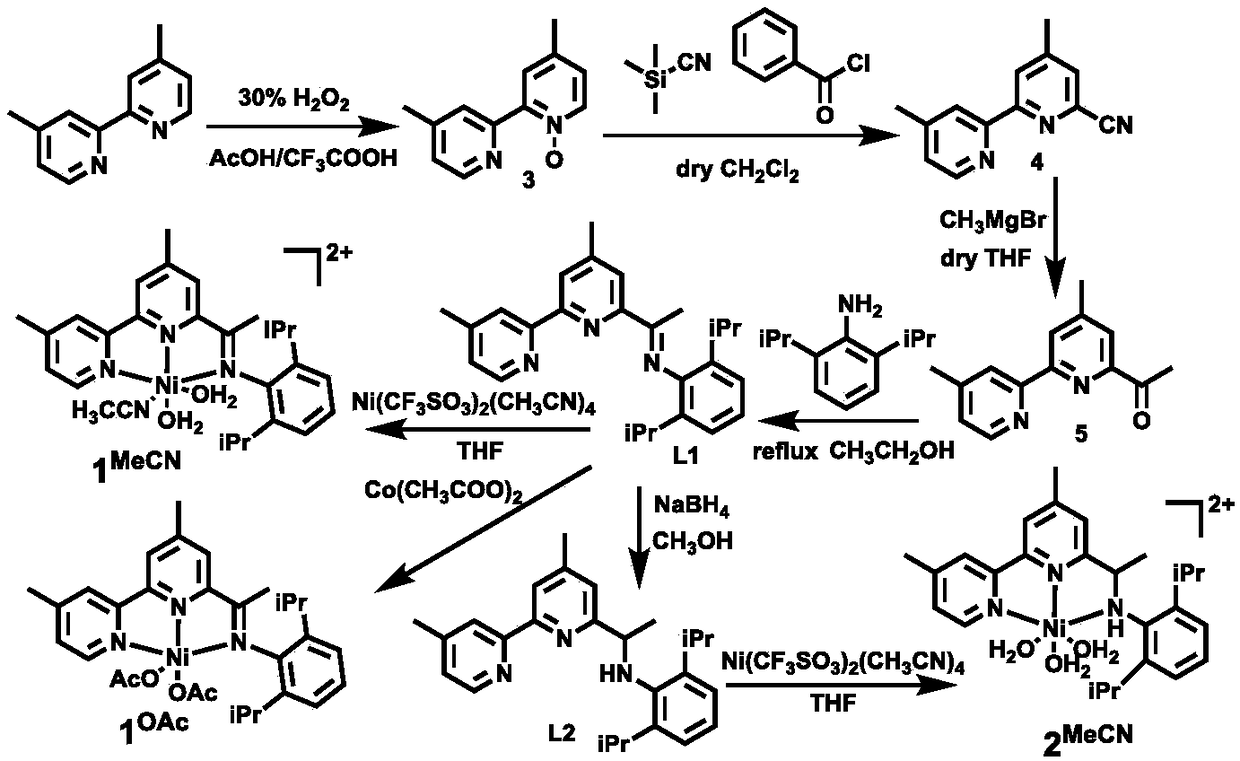
[0108] An asymmetric nitrogen-hydrogen-pyridine-nickel-acetonitrile metal catalyst, namely 2 MeCN , its structural formula is as follows:
[0109]
[0110] The synthetic route of above-mentioned catalyst is as figure 1 As shown, the preparation includes the following steps:
[0111] 1) Synthesis of Compound 5:
[0112] In compound 4 (1.05g, 5.0mmol) in dry tetrahydrofuran, methylmagnesium bromide (3.0M in ether solution, 0.85mL, 2.5 equivalents, 12.5mmol) was added dropwise at -15°C, and reacted at -15°C Stirring was continued for 1 h at room temperature, and then stirring was continued for 2 h at room temperature to obtain an orange-red liquid. After the reaction was completed, saturated ammonium chloride was added dropwise under ice bath conditions to quench the reaction, the organic phase was extracted with tetrahydrofuran and dichloromethane respectively, the organic phase was combined, washed three times with saturated brine, dried over anhydrous sodium sulfate, the...
Embodiment 2
[0132] 2 prepared in Example 1 MeCN Perform electrochemical performance characterization:
[0133] In 2.5mL of 0.1M tetrabutylammonium hexafluorophosphate acetonitrile, add the 2 prepared in Example 1 MeCN , to get a mixed solution, wherein the concentration of the mixed solution is 2mM (that is, every L tetrabutylammonium hexafluorophosphate acetonitrile contains 2mmol of 1 MeCN ), after 10 min of Ar, the electrochemical CV was scanned at a scan rate of 50 mV / s; after that, after 10 min of carbon dioxide, the electrochemical CV was scanned at a scan rate of 50 mV / s.
[0134] Characterization results such as Figure 5 Shown, the compound's cyclic voltammetry curve (CV) Ar or CO in acetonitrile solution 2 Obtained under; Among them, under Ar, compound 1 MeCN exhibits two reversible electrochemical peaks E 1 / 2 =-0.35V (peak I) and -1.03V (peak II), peak I is electrochemically reversible, which is attributed to Ni(II) / Ni(I) electrochemical reduction electron pair, peak II is...
Embodiment 3
[0143] Test the generation of intermediate compound on the influence of asymmetric nitrogen-hydrogen-pyridine-nickel-acetonitrile metal catalyst electrochemical performance, the steps are as follows:
[0144] In 2.5mL of 0.1M tetrabutylammonium hexafluorophosphate in acetonitrile, add the 2 prepared in Example 1 MeCN, to get a mixed solution, wherein the concentration of the mixed solution is 5mM (that is, every L tetrabutylammonium hexafluorophosphate acetonitrile contains 2mmol of 1 MeCN ), after passing 20minAr, after saturation, electrolyze at -1.45V vs. NHE for 4h. Measure liquid infrared and observe the result. Compare the results of electrolysis and CO under Ar without electrolysis 2 Below is the electrolysis result.
[0145] The result is as Figure 7 and Figure 8 As shown, under Ar, 2 MeCN Three new peaks appeared, corresponding to the C═O double bond vibration and the C—O single bond vibration of carbon dioxide coordination. Explain that Ni compound and CO 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com