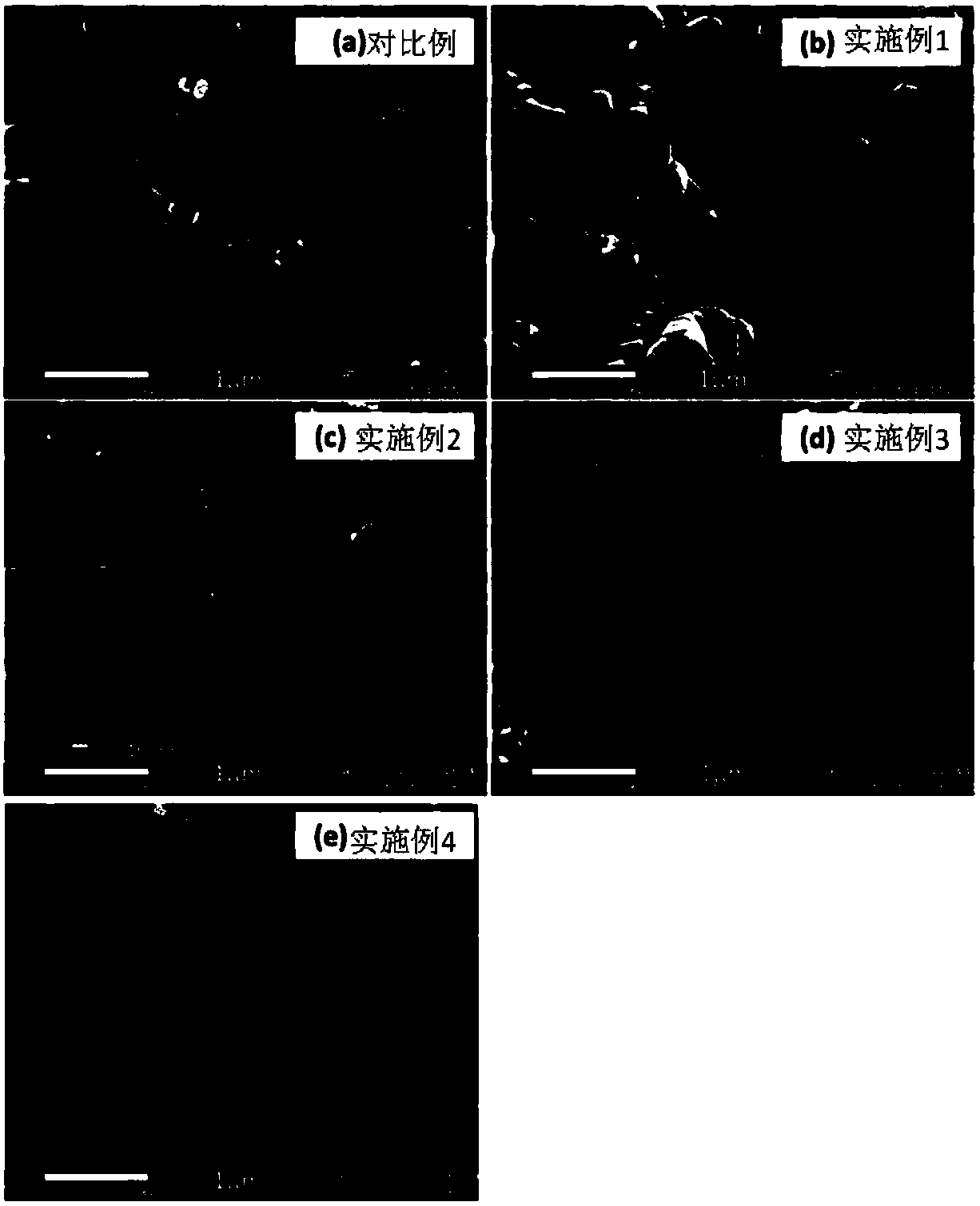
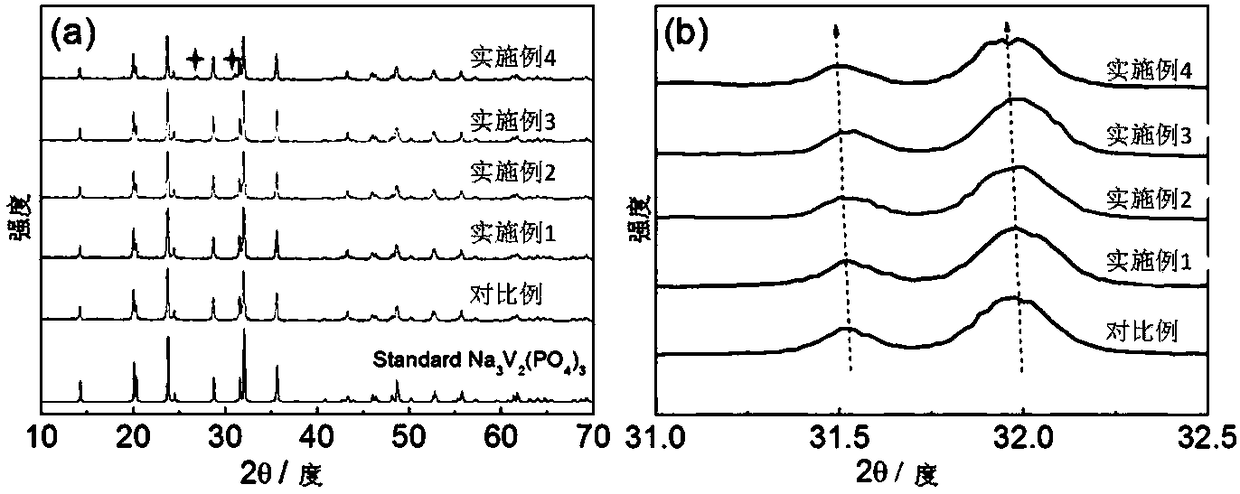
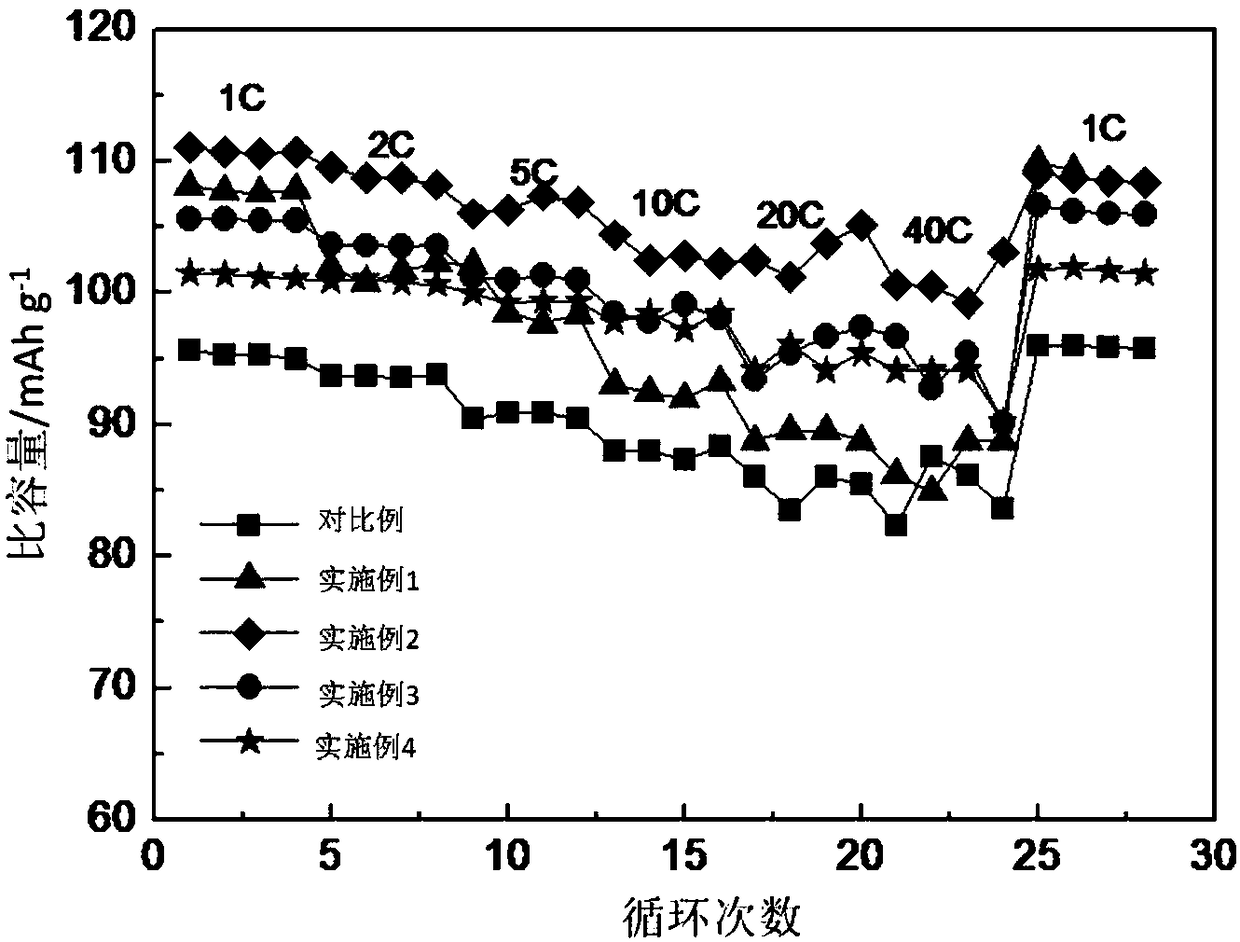
Rare earth metal doped cathode material for sodium-ion battery as well as preparation and application of cathode material
A sodium-ion battery and cathode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of no improvement in electrical conductivity and achieve the effect of promoting electron conduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: (Na 3 V 1.98 Ce 0.02 (PO 4 ) 3 )
[0025] The mass of sodium hydroxide, ammonium metavanadate, cerium nitrate and ammonium dihydrogen phosphate is weighed in a molar ratio of 3:1.98:0.02:2, and both the reducing agent and the carbon source are selected from citric acid as an example. First, ammonium metavanadate , cerium nitrate, and citric acid in a molar ratio of 1.98:0.02:2 (wherein 40.46% is a reducing agent and 59.54% is a carbon source) and 250ml of deionized water are mixed, stirred and heated in a water bath (80°C) to form a well-mixed For the blue solution, add weighed sodium hydroxide and ammonium dihydrogen phosphate into the above blue solution, and stir to form a sol. The sol was then rotary evaporated at 75° C. for 1 hour under vacuum condition to form a gel. Subsequently, the gel was dried in a vacuum oven at 130°C for 10 h to form Na 3 V 1.98 Ce 0.02 (PO 4 ) 3 Precursor. The precursor is pre-sintered at 350°C for 5 hours under i...
Embodiment 2
[0026] Embodiment 2: (Na 3 V 1.96 Ce 0.04 (PO 4 ) 3 )
[0027] The mass of sodium hydroxide, ammonium metavanadate, cerium nitrate and ammonium dihydrogen phosphate is weighed in a molar ratio of 3:1.96:0.04:2. Both the reducing agent and the carbon source are selected from citric acid as an example. First, ammonium metavanadate , cerium nitrate, and citric acid in a molar ratio of 1.96:0.04:2 (wherein 40.05% is a reducing agent and 59.95% is a carbon source) and 250ml of deionized water are mixed, stirred and heated in a water bath (80°C) to form a well-mixed For the blue solution, add weighed sodium hydroxide and ammonium dihydrogen phosphate into the above blue solution, and stir to form a sol. The sol was then rotary evaporated at 75° C. for 1 hour under vacuum condition to form a gel. Subsequently, the gel was dried in a vacuum oven at 130°C for 10 h to form Na 3 V 1.96 Ce 0.04 (PO 4 ) 3 Precursor. The precursor is pre-sintered at 350°C for 5 hours under inert...
Embodiment 3
[0028] Embodiment 3: (Na 3 V 1.94 Ce 0.06 (PO 4 ) 3 )
[0029] The mass of sodium hydroxide, ammonium metavanadate, cerium nitrate and ammonium dihydrogen phosphate is weighed in a molar ratio of 3:1.94:0.06:2. Both the reducing agent and the carbon source are selected from citric acid as an example. First, ammonium metavanadate , cerium nitrate, citric acid in a molar ratio of 1.94:0.06:2 (wherein 39.64% is a reducing agent and 60.36% is a carbon source) and 250ml of deionized water are mixed, stirred and heated in a water bath (80°C) to form a uniform mixture For the blue solution, add weighed sodium hydroxide and ammonium dihydrogen phosphate into the above blue solution, and stir to form a sol. The sol was then rotary evaporated at 75° C. for 1 hour under vacuum condition to form a gel. Subsequently, the gel was dried in a vacuum oven at 130°C for 10 h to form Na 3 V 1.94 Ce 0.06 (PO 4 ) 3 Precursor. The precursor is pre-sintered at 350°C for 5 hours under iner...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com