Novel tetracarboxylic dianhydride, polyimide derived from said tetracarboxylic dianhydride, and molded article produced from said polyimide
A technology of tetracarboxylic dianhydride and polyimide is applied in the field of novel tetracarboxylic dianhydride and polyimide derived from the tetracarboxylic dianhydride and the formed body composed of the polyimide, which can solve the problem of dissolving Insufficient properties, undiscovered polyimide, etc., to achieve the effect of excellent solubility and excellent processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The synthesis of tetraformic acid dianhydride TACHQ shown in A. formula (1)
[0110] TACHQ represented by formula (1) is synthesized as follows. 12.7003 g (60.3137 mmol) of trimellitic anhydride chloride was placed in an eggplant-shaped flask, dissolved in 33 mL of dehydrated tetrahydrofuran (THF) at room temperature, and sealed with a septum to prepare Solution A. Further, 6.5 mL of dehydrated THF and 9.7 mL (120 mmol) of pyridine were added to 3.8551 g (20.0661 mmol) of 2-cyclohexylhydroquinone (CHQ) in another flask, and a septum was sealed to prepare solution B.
[0111] While cooling and stirring in an ice bath, solution B was slowly added dropwise to solution A via a syringe over about 5 minutes, followed by stirring at room temperature for 24 hours. After the reaction, the white precipitate was filtered and washed with THF and ion-exchanged water. The removal of pyridine hydrochloride was confirmed by adding an aqueous solution of silver nitrate to the washing ...
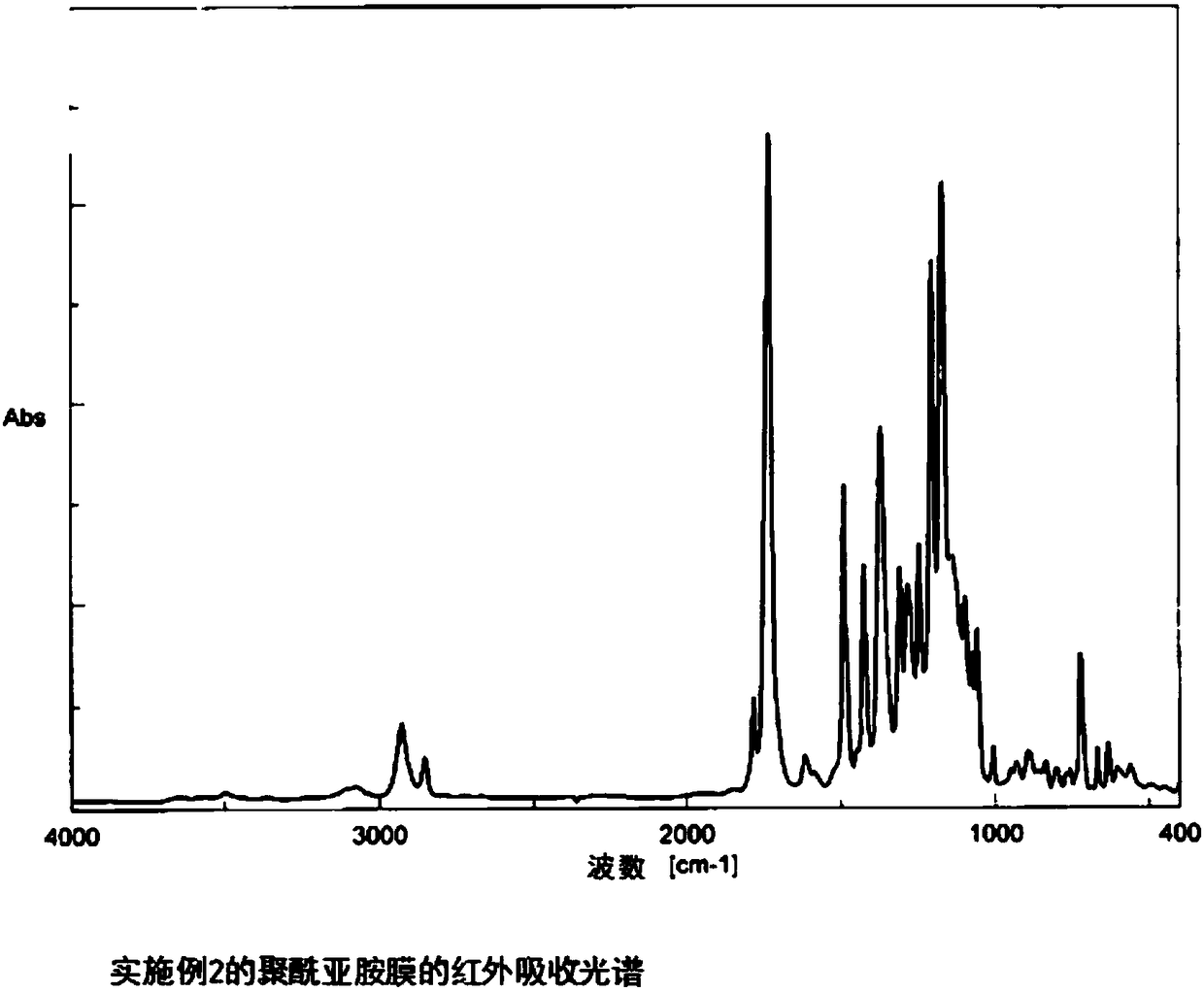
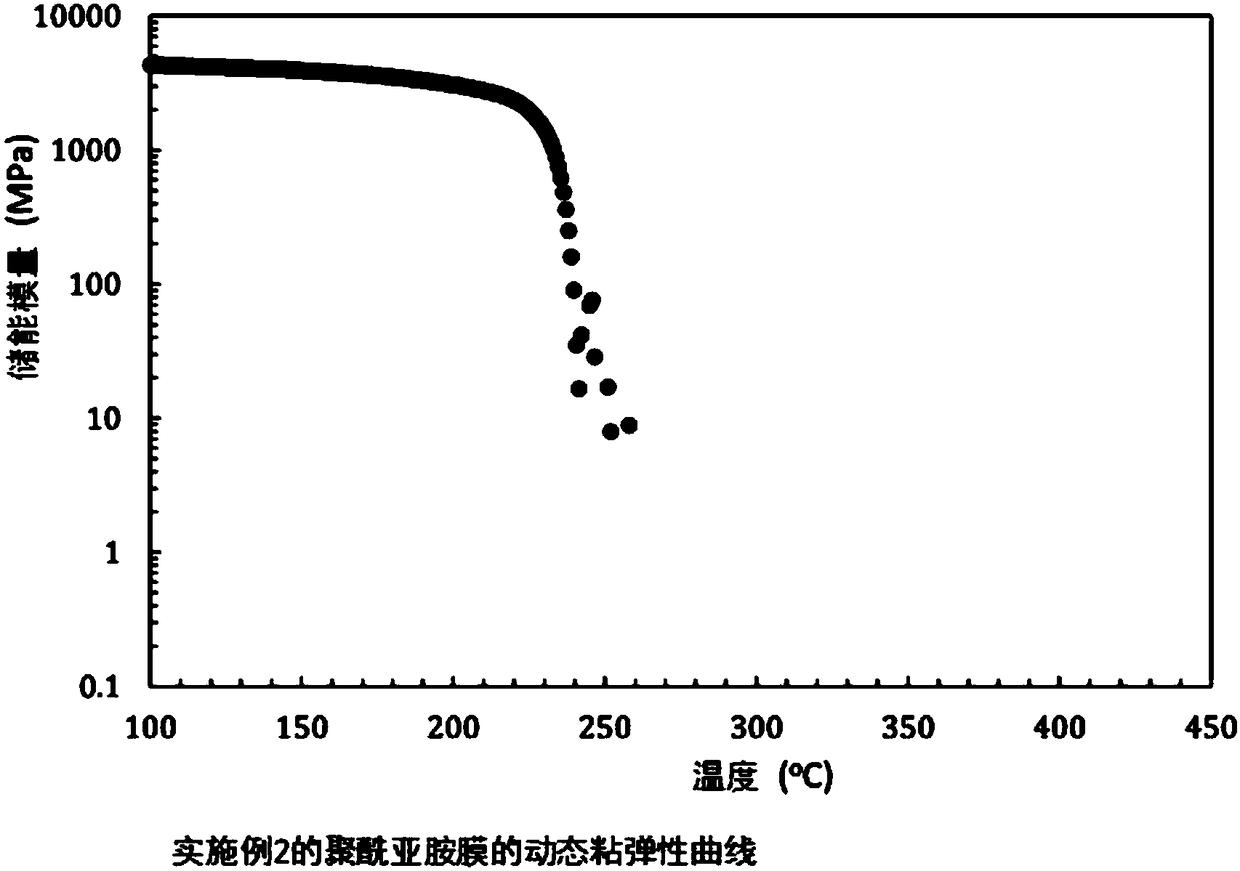
Embodiment 2
[0120] The synthesis of the polyimide of the repeating unit shown in A. formula (8)
[0121] (Polymerization of polyamic acid) TACHQ / TFMB
[0122] 3 mmol of 2,2'-bis(trifluoromethyl)benzidine (TFMB) was dissolved in dehydrated N,N-dimethylacetamide (DMAc). 3 mmol of the TACHQ powder described in Example 1 was slowly added there, it stirred at room temperature for 72 hours, and DMAc was added suitably, and the polyamic acid (16.7 weight% of solid content concentration) which is a polyimide precursor was obtained. The intrinsic viscosity of the obtained polyamic acid was 1.72 dL / g.
[0123] (Chemical imidization reaction)
[0124] After diluting the obtained polyamic acid solution to a solid content concentration of about 10.0% by weight with dehydrated DMAc, a mixture of 2.8 mL (30 mmol) of acetic anhydride and 1.2 mL (15 mmol) of pyridine was slowly added dropwise at room temperature while stirring. The solution was mixed and further stirred for 24 hours after the dropwise ...
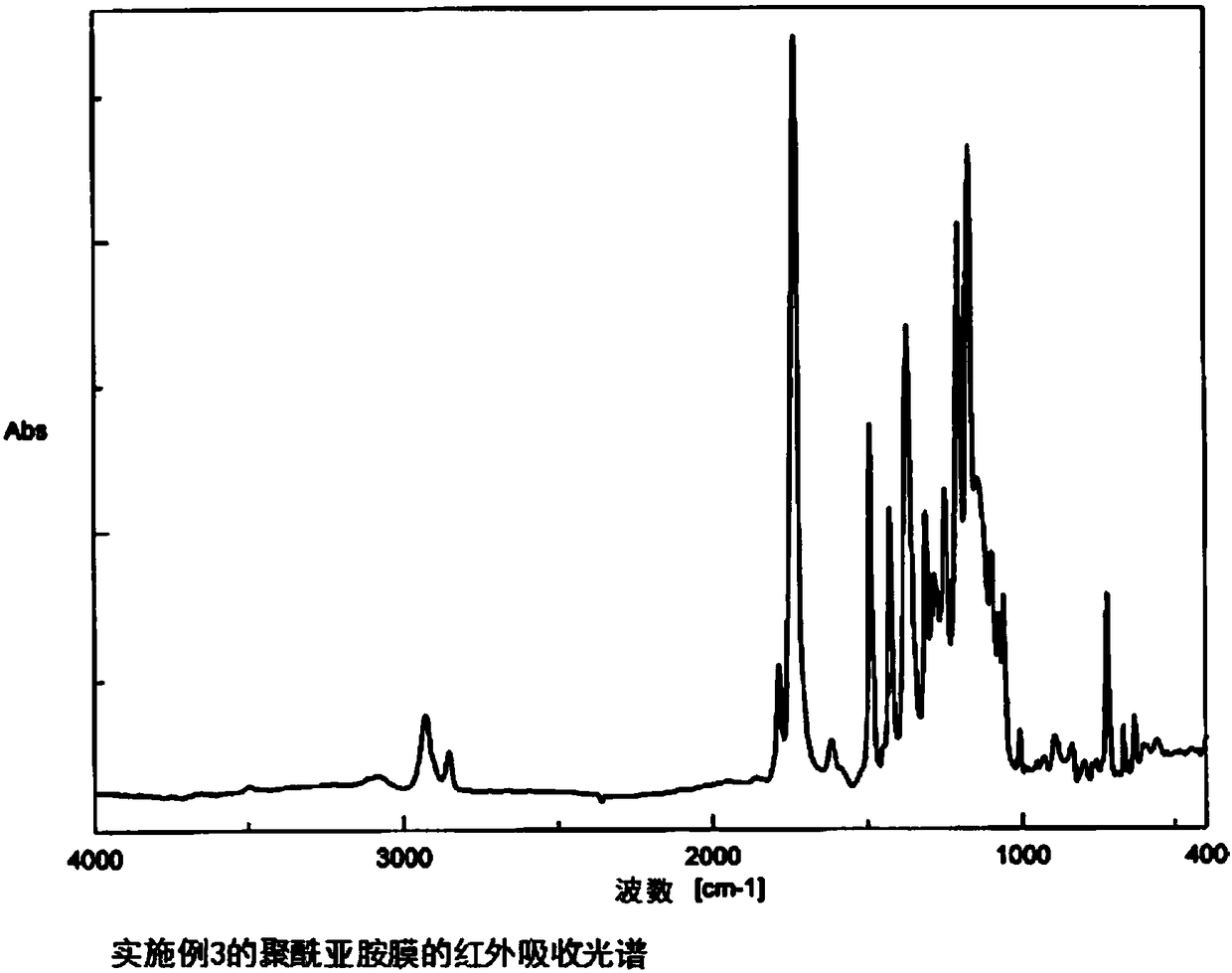
Embodiment 3
[0130] A. Synthesis of the polyimide of the repeating unit represented by the following formula (9)
[0131] (Polymerization of polyamic acid) TACHQ(80)6FDA(20) / TFMB
[0132]3 mmol of 2,2'-bis(trifluoromethyl)benzidine (TFMB) was dissolved in dehydrated N,N-dimethylacetamide (DMAc). Slowly add 2.4 mmol of TACHQ powder described in Example 1 and 0.6 mmol of 4,4'-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) powder, stir at room temperature for 72 hours, and add DMAc was used to obtain polyamic acid (solid content concentration: 22.7% by weight) which is a polyimide precursor. The intrinsic viscosity of the obtained polyamic acid was 0.91 dL / g.
[0133] (Chemical imidization reaction)
[0134] After diluting the obtained polyamic acid solution with dehydrated DMAc to a solid content concentration of about 10.0% by weight, a mixture of 2.8 mL (30 mmol) of acetic anhydride and 1.2 mL (15 mmol) of pyridine was slowly added dropwise at room temperature while stirring. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com