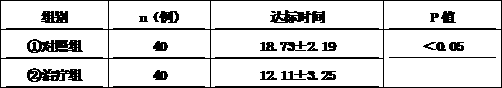
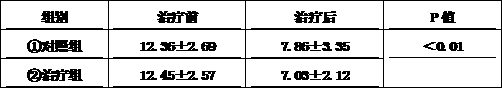Medicine composition used for treating diabetes mellitus
A composition and technology for diabetes, applied in drug combinations, metabolic diseases, pharmaceutical formulations, etc., to achieve the effects of improving insulin resistance, protecting islet cell function, and lowering cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0039] Embodiment: The pharmaceutical composition is made up of following raw material:
[0040] Eupatorium extract 330g, sealwort 330g, ginseng 170g, bitter melon 990g, wild papaya 650g, chayote 330g and metformin 500g.
[0041] (1) Dry and pulverize ginseng, sealwort, wild papaya, bitter gourd and chayote at 45-50°C respectively to 120-mesh fine powder;
[0042] (2) Take Eupatorium, add 4 to 6 times the amount of water each time, decoct twice, the first time is 1.5 to 2 hours, and the second time is 0.5 to 1 hour, combine the two decoctions, filter, and concentrate the filtrate At 80-85°C, add ethanol to the clear ointment with a relative density of 1.08-1.10, so that the alcohol content of the clear ointment reaches 70%, let it stand for 24 hours, recover ethanol, and concentrate at 50-55°C to a relative density of 1.30-1.32 The clear ointment, dried, Zelan extract, and then crushed into 120 mesh fine powder;
[0043] (3) Mix metformin with the fine powder prepared in (1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com