Magnesium oxide-supported ruthenium catalyst for hydrogen preparation through ammonia decomposition, preparation method and applications thereof
A technology of ruthenium catalyst and magnesium oxide, which is applied in the field of preparation of highly active magnesia-supported ruthenium catalyst, can solve the problems of different preparation methods and catalyst performance differences, and achieve the effect of simple preparation process, low production cost and uniform dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
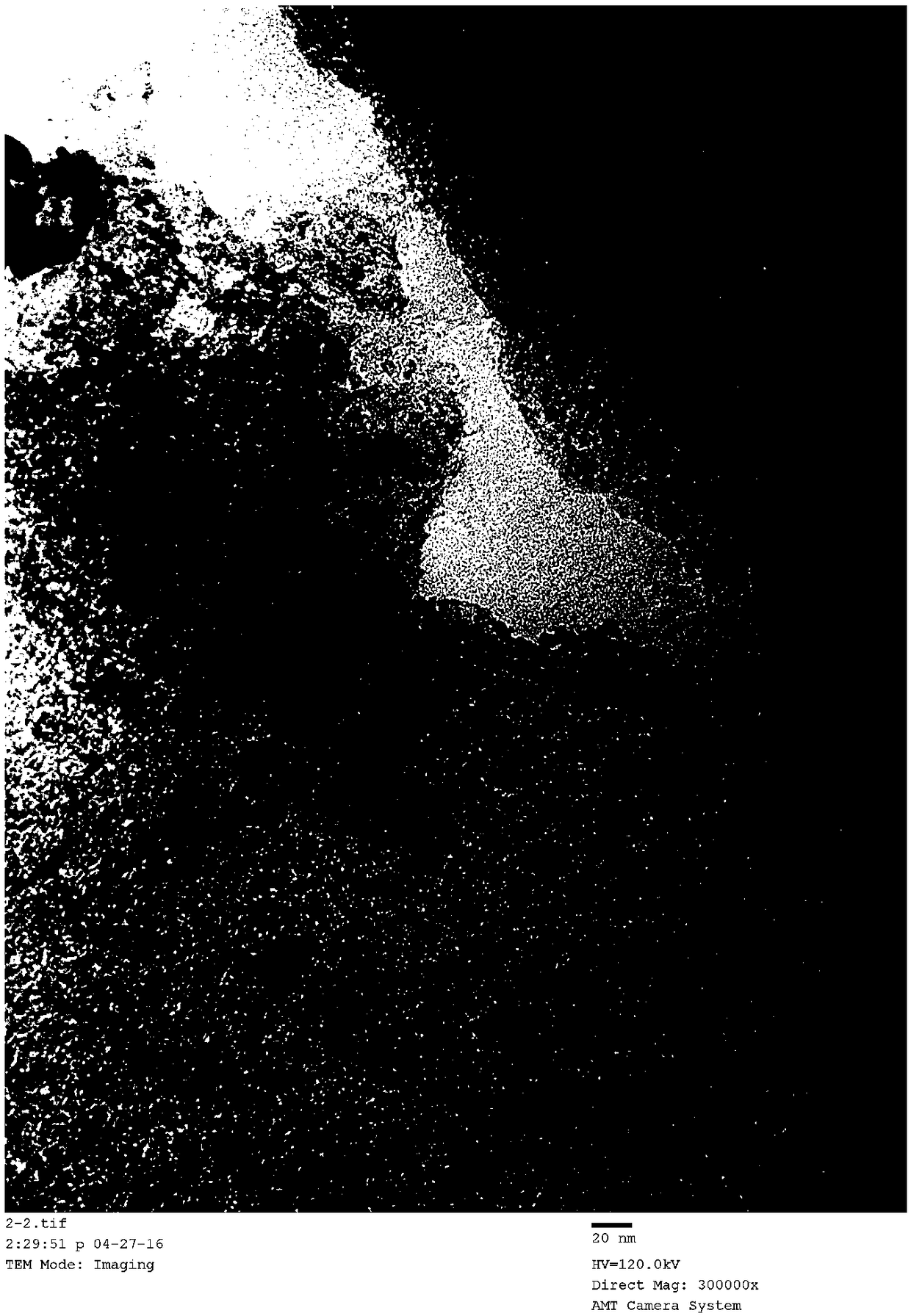
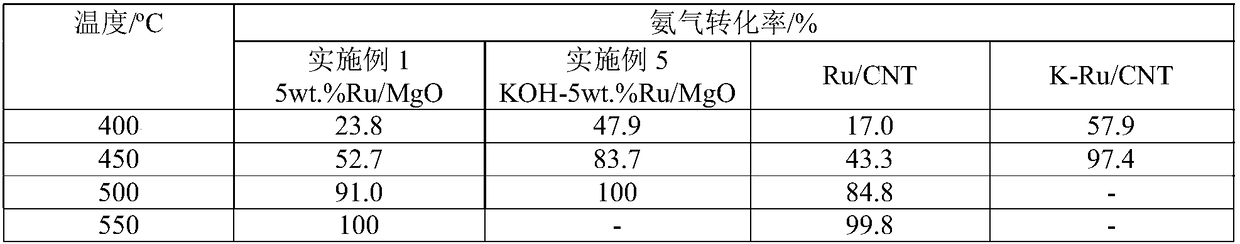
Image
Examples
Embodiment 1
[0027] Take by weighing 0.0513 gram of ruthenium chloride and be dissolved in 60ml water, under stirring, 0.5 gram of commercial magnesia (specific surface is 20m 2 / g) was added to the aqueous ruthenium chloride solution, stirred evenly, and then 3.0 g of urea was added to the suspension, and then refluxed at 80° C. for 8 hours and room temperature for 12 hours under stirring. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. The product was dried at 80°C for 6 hours, and then reduced with ammonia gas at 500°C for 2 hours to obtain a 5 wt.% Ru / MgO catalyst.
Embodiment 2
[0029] Take by weighing 0.0308 gram of ruthenium chloride and be dissolved in 60ml water, under stirring, 0.5 gram of commercial magnesia (specific surface is 50m 2 / g) was added to an aqueous ruthenium chloride solution, stirred evenly, and then 1.0 g of urea was added to the suspension, and then refluxed at 120° C. for 2 hours and room temperature for 12 hours under stirring. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. The product was dried at 60°C for 24 hours to prepare a granular catalyst. The above solid particles were filled into a reactor, and then reduced with ammonia gas at 500°C for 4 hours to obtain a reduced 3wt.%Ru / MgO catalyst. In the reduced magnesium oxide-supported ruthenium catalyst, potassium hydroxide is used as an auxiliary agent to modify the magnesium oxide-supported ruthenium catalyst. With respect to the amount of ruthenium, the molar ratio of the added amount of potassi...
Embodiment 3
[0031] Take by weighing 0.0205 gram of ruthenium chloride and be dissolved in 60ml water, under stirring, 0.5 gram of commercial magnesia (specific surface is 100m 2 / g) was added to the ruthenium chloride aqueous solution, stirred evenly, and then 0.2 g of urea was added to the suspension, and then refluxed at 90°C for 12 hours and room temperature for 12 hours under stirring. After the reaction is completed, filter and wash the product repeatedly with deionized water until the filtrate is neutral. The product was dried at 130°C for 24 hours to prepare a granular catalyst. The above solid particles were filled into a reactor, and then reduced with ammonia gas at 200° C. for 12 hours to obtain a reduced 2wt.% Ru / MgO catalyst. In the reduced magnesium oxide-supported ruthenium catalyst, potassium carbonate is used as an auxiliary agent to modify the magnesium oxide-supported ruthenium catalyst. Relative to the amount of ruthenium, the molar ratio of the amount of potassium ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com