A kind of mycoplasma bovis drug susceptibility rapid detection kit and preparation method thereof
A detection kit and technology for Mycoplasma bovis, applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection, etc., can solve the problems of no commercialized kits, no national standards or patents, etc. Shorter color development time, high efficiency, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In a second aspect, the present invention provides a preparation method of the Mycoplasma bovis drug susceptibility rapid detection kit, comprising the following steps:
[0030] (1) Preparation of Mycoplasma bovis culture solution: dissolve 12 g of PPLO powder, 3 g of short peptide, 300 mg of morroniside, 4 g of yeast powder, 2.5 g of glucose, and 3.5 g of sodium pyruvate in 750 mL of ddH 2 O, add 100 mL of MEM medium, 2 mL of 1% (w / v) phenol red solution, sterilize at 116 °C for 20 min, add 150 mL of horse serum inactivated at 56 °C and 300,000 units of penicillin, and adjust the pH to 7.6 to 7.8 , dispensed into 15mL culture tubes, 9mL / tube.
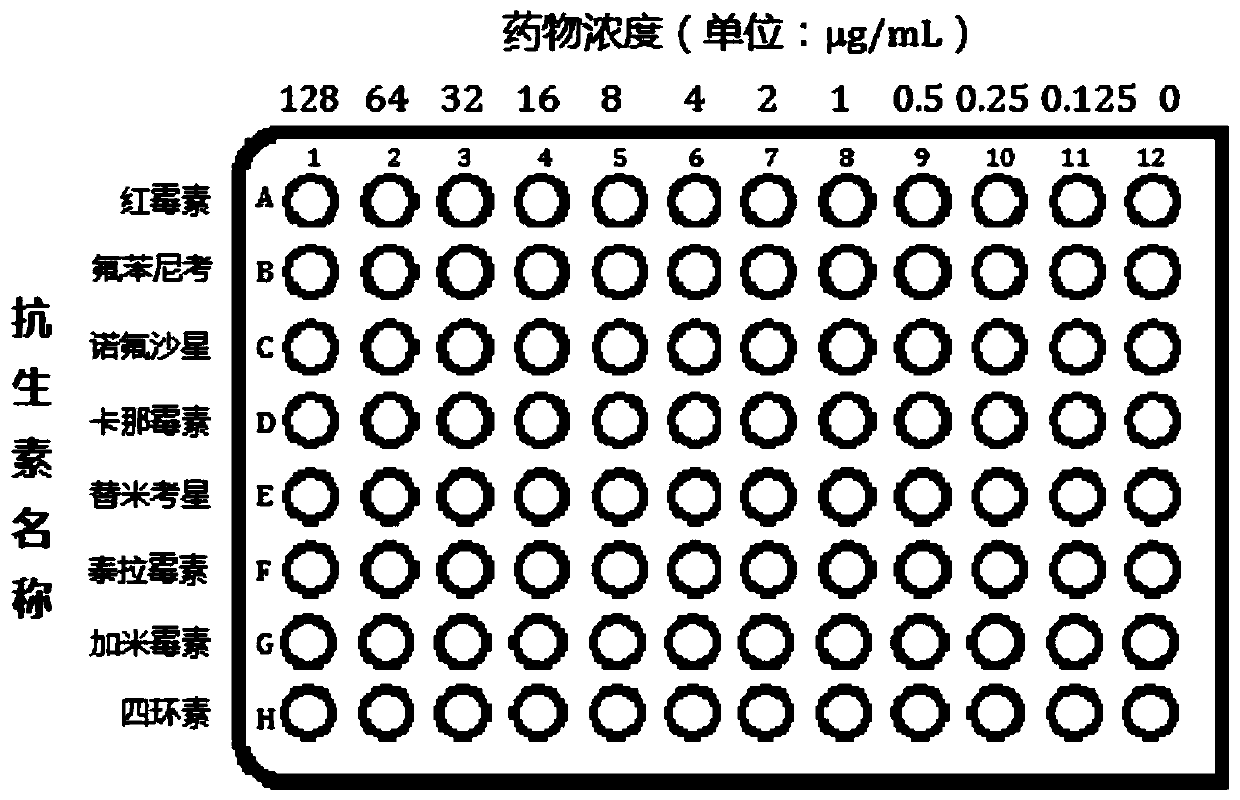
[0031] (2) Preparation of drug sensitive plate:
[0032] S1. The 96-well plate was washed roughly, finely washed, and dried at a constant temperature of 40°C for use;
[0033] S2. Dissolve erythromycin, florfenicol, norfloxacin, kanamycin, tilmicosin, teramycin, gamycin and tetracycline into 1-5 mL buffer, and dilute to 128μg...
Embodiment 1
[0036] Embodiment 1 A kind of Mycoplasma bovis culture solution
[0037] The Mycoplasma bovis culture solution is composed of the following components and contents: 10-15 g of PPLO powder, 2-4 g of short peptide, 100-500 mg of morroniside, 2-6 g of yeast powder, 1-3 g of glucose, and 1.5-4.5 g of sodium pyruvate , MEM medium 80~120mL, 1% (w / v) phenol red solution 2mL, horse serum 100~300mL, 300,000 units of penicillin and ddH 2 O 700~800mL.
[0038] The peptide chain is an oligomeric short peptide of Tyr-Leu-Tyr-Glu-Val-Ala.
Embodiment 2
[0039] Embodiment 2 A kind of Mycoplasma bovis culture solution
[0040] The Mycoplasma bovis culture solution is composed of the following components and contents: 10-15 g of PPLO powder, 2-4 g of short peptide, 100-500 mg of morroniside, 2-6 g of yeast powder, 1-3 g of glucose, and 1.5-4.5 g of sodium pyruvate , MEM medium 80~120mL, 1% (w / v) phenol red solution 2mL, horse serum 100~300mL, 300,000 units of penicillin and ddH 2 O 700~800mL.
[0041] The peptide chain is an oligomeric short peptide of Tyr-Leu-Tyr-Glu-Val-Ala.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com