Preparation method and application of chiral bonded capillary electrochromatography open tubular column
A technology of capillary electrochromatography and open-tube columns, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of easy detachment of stationary phase and short service life, so as to improve mechanical strength and stability, increase sample capacity, increase Effect of Column Efficiency and Separation Selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
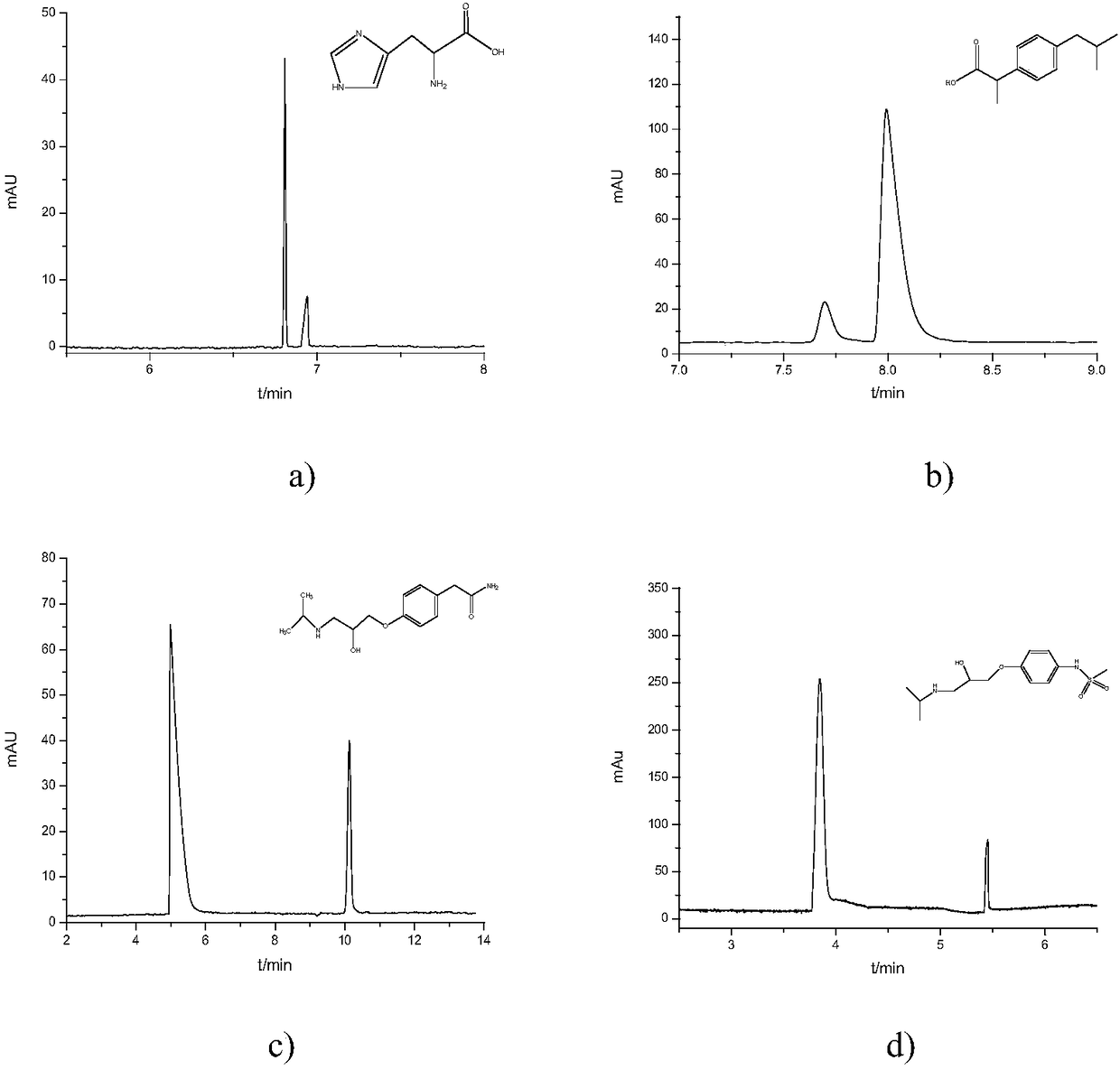
Examples
preparation example Construction
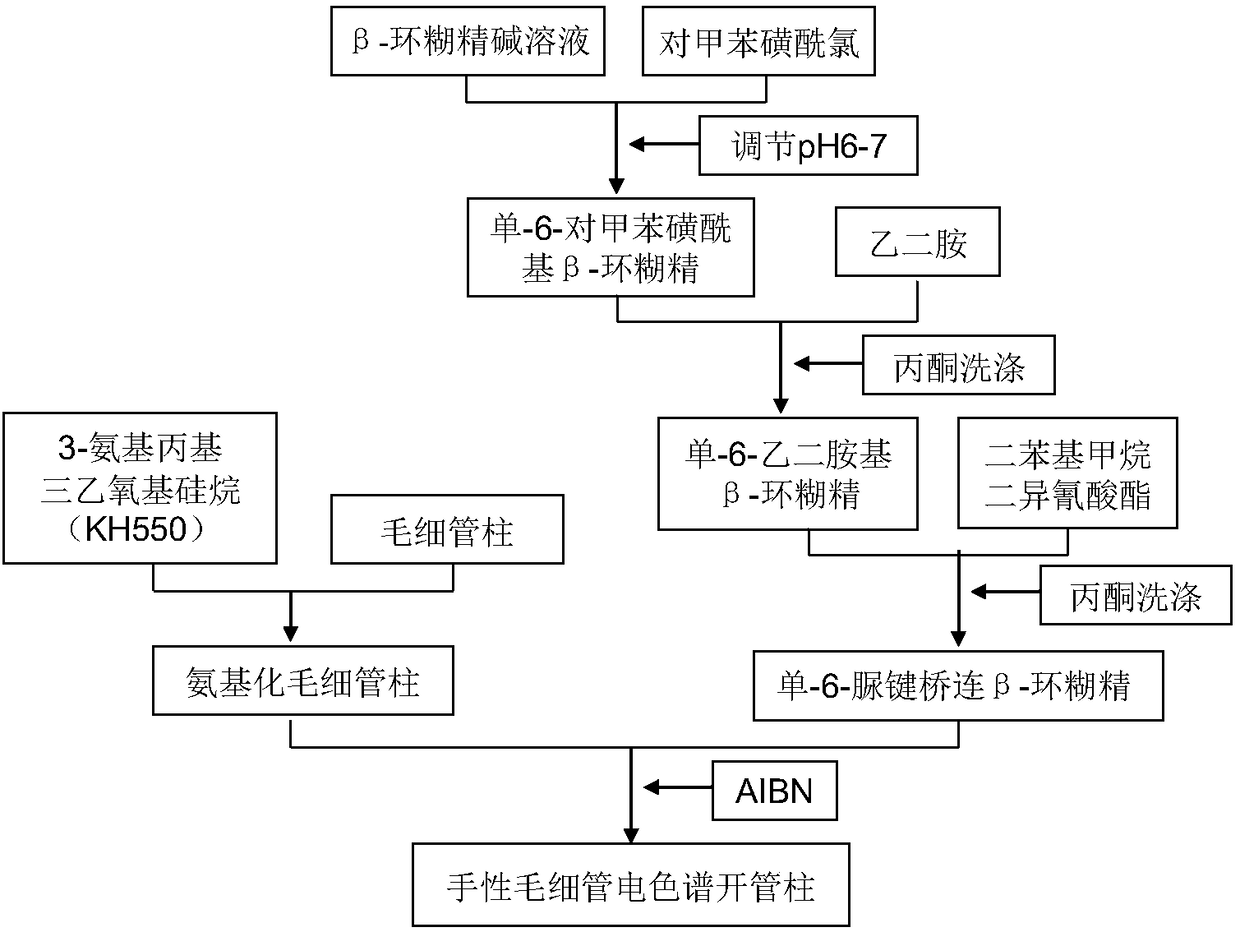
[0029] The invention provides a preparation method of a chiral bonded capillary electrochromatographic open column, the process flow is as follows figure 1 As shown, the specific steps are:
[0030] (1) Preparation of single-6-p-toluenesulfonyl β-cyclodextrin:
[0031] Dissolve 5.0g of β-cyclodextrin (β-CD) in 100mL of 30% sodium hydroxide solution, add 1.5-1.8g of p-toluenesulfonyl chloride in a cold water bath, stir for 4-6 hours, filter, and use 1mol / L HCl solution adjusted the pH of the filtrate to 6-7, and a white precipitate precipitated out. The mixture was placed in a refrigerator (0-4°C) overnight, filtered, and the precipitate was recrystallized twice with distilled water, acetonitrile:water (volume ratio 1:1) Recrystallize once and dry under vacuum at 50°C to obtain mono-6-p-toluenesulfonyl β-cyclodextrin (6-OTs-β-CD).
[0032] (2) Preparation of mono-6-ethylenediamino-β-cyclodextrin
[0033] Weigh 3.0g 6-OTs-β-CD, add 40~50mL ethylenediamine, stir to dissolve, r...
Embodiment 1
[0047] In this embodiment, the preparation method of chiral bonded capillary electrochromatography open-tubular column, the specific steps are as follows:
[0048] (1) Preparation of single-6-p-toluenesulfonyl β-cyclodextrin:
[0049] Dissolve 5.0g of β-CD in 100mL of 30% sodium hydroxide solution, add 1.6g of p-toluenesulfonyl chloride in a cold water bath, stir for 5 hours, filter, and adjust the pH of the filtrate to 6-7 with 1mol / L HCl solution. Precipitation was precipitated, the mixture was placed in a refrigerator (0-4°C) overnight, filtered, the precipitate was recrystallized twice with distilled water, acetonitrile:water (volume ratio 1:1) was recrystallized once, and vacuum-dried at 50°C to obtain a single - 6-p-toluenesulfonyl β-cyclodextrin (6-OTs-β-CD).
[0050] (2) Preparation of mono-6-ethylenediamino-β-cyclodextrin
[0051] Weigh 3.0g of 6-OTs-β-CD, add 45mL of ethylenediamine, stir to dissolve, react at 80°C for 4h, add acetone to precipitate a white precipi...
Embodiment 2
[0068] In this embodiment, the preparation method of chiral bonded capillary electrochromatography open-tubular column, the specific steps are as follows:
[0069] (1) Preparation of single-6-p-toluenesulfonyl β-cyclodextrin:
[0070] Dissolve 5.0g of β-CD in 100mL of 30% sodium hydroxide solution, add 1.6g of p-toluenesulfonyl chloride in a cold water bath, stir for 6 hours, filter, adjust the pH of the filtrate to 6-7 with 1mol / L HCl solution, and have a white Precipitation was precipitated, the mixture was placed in a refrigerator (0-4°C) overnight, filtered, the precipitate was recrystallized twice with distilled water, acetonitrile:water (volume ratio 1:1) was recrystallized once, and vacuum-dried at 50°C to obtain a single - 6-p-toluenesulfonyl β-cyclodextrin (6-OTs-β-CD).
[0071] (2) Preparation of mono-6-ethylenediamino-β-cyclodextrin
[0072] Weigh 3.0g of 6-OTs-β-CD, add 45mL of ethylenediamine, stir to dissolve, react at 80°C for 4h, add acetone to precipitate a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com