Binuclear dysprosium cluster compound taking 2-aldehyde-8-hydroxyquinoline ethanolamine schiff base as ligand as well as synthesis methods and application thereof
A technology of ethanolamine Schiff base and hydroxyquinoline, which is applied in the direction of 3/13 organic compounds without C-metal bonds, compounds containing group 3/13 elements of the periodic table, chemical instruments and methods, etc., can solve the inconvenience Problems such as modeling and calculation, to achieve the effect of simple synthesis method, good repeatability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: The compound shown in formula (I) is the synthesis of 2-formyl-8-hydroxyquinoline ethanolamine Schiff base ligand
[0033]
[0034] The specific synthesis method is: weigh 0.0173g (0.1mmol) of 2-formyl-8-hydroxyquinoline and dissolve it in 5mL of methanol, and dissolve 0.061g (1mmol) of ethanolamine in 5mL of acetonitrile. The methanol solution of -8-hydroxyquinoline was slowly added to the acetonitrile solution of ethanolamine. At this time, the mixed solution was red. Put it into a magnet and stir for 20 minutes. The solution was light yellow, and a solution containing the ligand was obtained.
Embodiment 2
[0035] Embodiment 2: the compound shown in formula (I) is the synthesis of 2-formyl-8-hydroxyquinoline ethanalamine Schiff base ligand
[0036] Repeat Example 1, the difference is that the amount of methanol is changed to 2mL, the amount of acetonitrile is changed to 4mL, and the reaction time is changed to 40min.
Embodiment 3
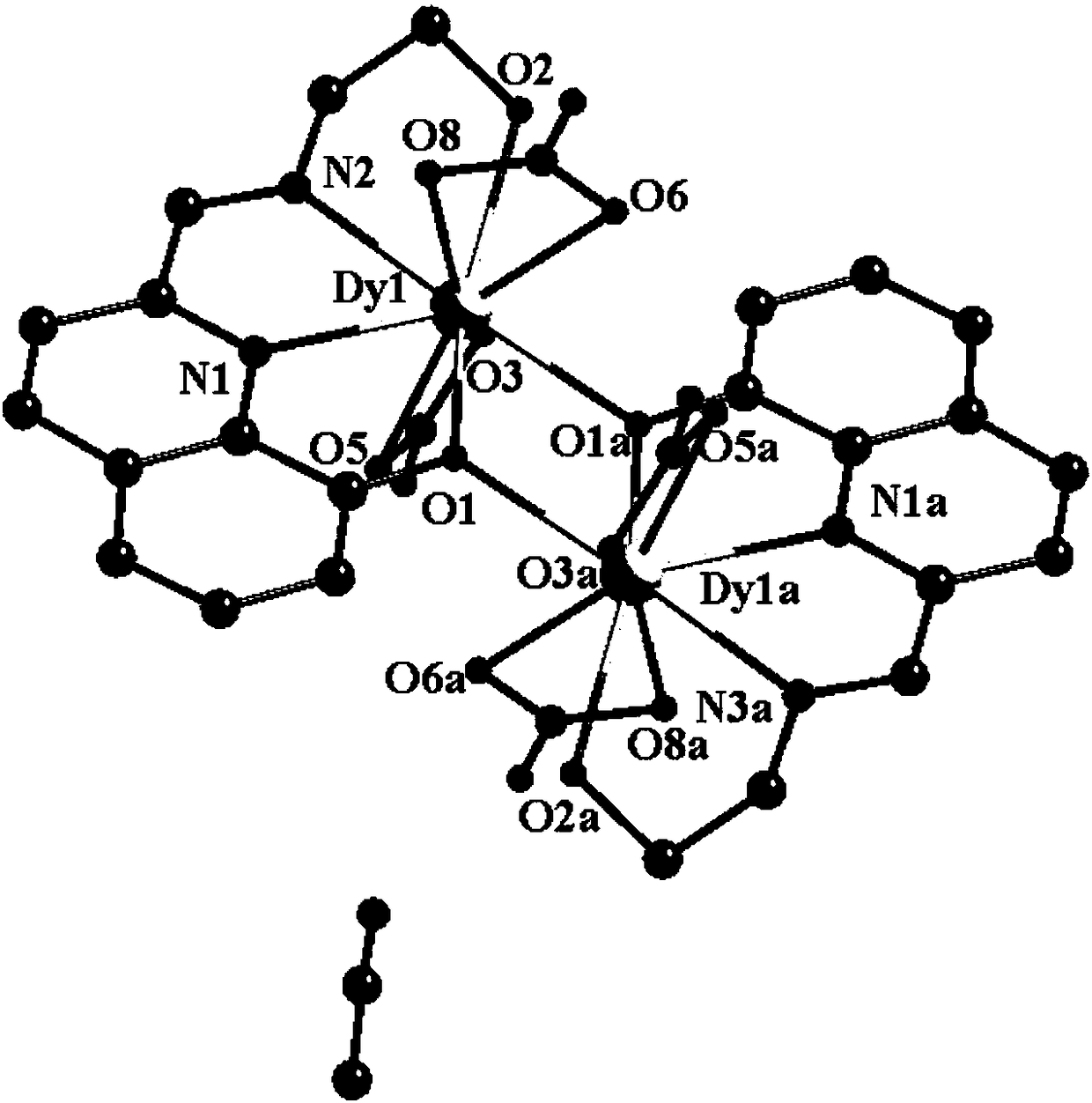
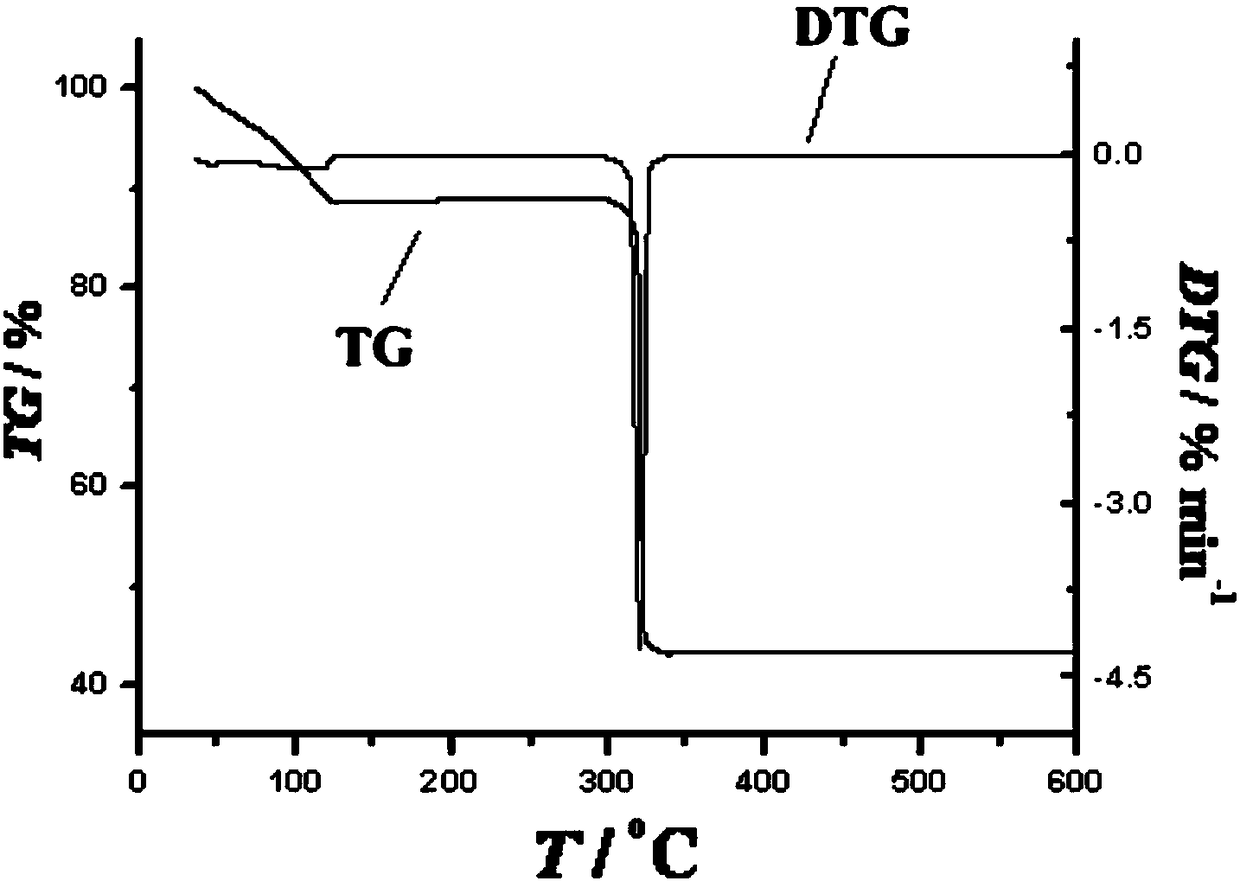
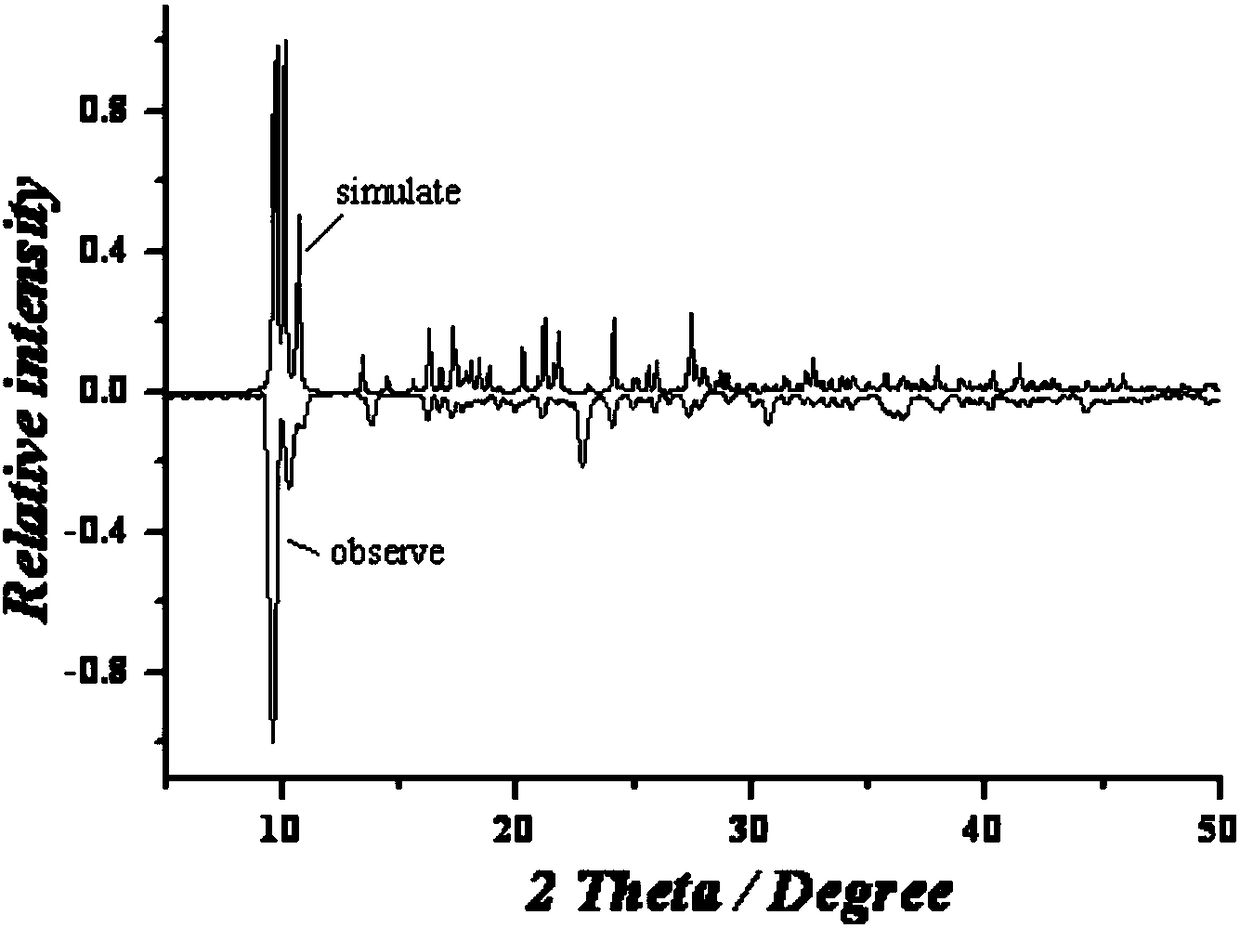
[0037] Embodiment 3: binuclear dysprosium cluster [Dy 2 (C 12 h 11 N 2 o 2 ) 2 (NO 3 ) 4 ]·CH 3 Synthesis of CN
[0038] Weigh Dy(NO 3 ) 3 ·6H 2 O (0.2mmol, 91.2mg) was placed in a glass bottle with a cover, and the ligand solution prepared in Example 1 was sucked with a disposable rubber tip dropper (1mL ligand solution contained methanol 0.5mL, acetonitrile 0.5mL, Ligand (0.1mmol) was added to the glass bottle, and then 0.5mL of methanol and 1.5mL of acetonitrile were added to it, so that the volume ratio of methanol and acetonitrile in the mixed solvent was 1:2, and then 22 μL of triethylamine was added to it after dissolving , shake gently until the precipitate disappears (the pH of the solution at this time is 6.7), then wrap the mouth of the bottle tightly with aluminum foil, cover the bottle cap, place the glass bottle at 60°C for 12 hours, take it out, and wrap it with cotton wool After the glass bottle was cooled to room temperature, yellow blocky crystals...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com