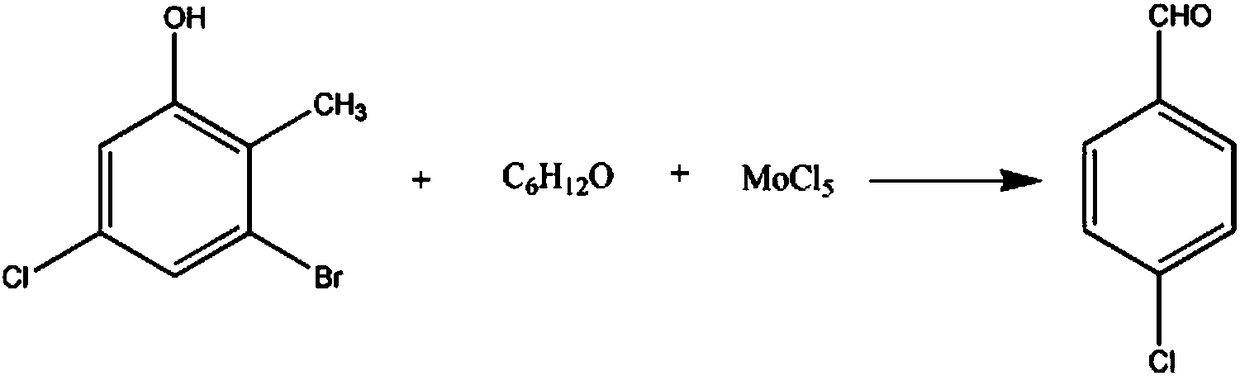Chlormezanone drug intermediate 4-chlorobenzaldehyde synthesis method
A technology of chlorobenzaldehyde and synthesis method, which is applied in the fields of oxidative preparation of carbonyl compounds, organic chemistry, etc., can solve problems such as high reaction temperature, high risk factor, and poisonous chlorine gas, and achieve low reaction temperature, lower risk factor, and high reaction yield Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthetic method of fenalu pharmaceutical intermediate 4-chlorobenzaldehyde comprises the steps:
[0016] Add 2-methyl-3-bromo-5-chlorophenol of 2.5mol, 3mol mass fraction and be 80% 4-methyl-2-pentanone solution in the reaction vessel, control stirring speed 170rpm, raise solution temperature to 40°C, add 3.2mol molybdenum pentachloride powder in 3 times, continue to react for 40min after addition, 1.32kPa vacuum distillation, collect 82°C fraction, wash in 80% butyl oxalate solution for 3 Washing 5 times in 85% ethanedinitrile solution and recrystallizing in 90% 2-methylbutyric acid solution gave 311.5 g of crystalline 4-chlorobenzaldehyde with a yield of 89%.
Embodiment 2
[0018] The synthetic method of fenalu pharmaceutical intermediate 4-chlorobenzaldehyde comprises the steps:
[0019] Add 2.5mol of 2-methyl-3-bromo-5-chlorophenol and 3.3mol mass fraction of 84% 4-methyl-2-pentanone solution in the reaction vessel, control the stirring speed to 190rpm, and raise the solution temperature To 45°C, add 3.4mol molybdenum pentachloride powder in 5 times, continue to react for 45min after addition, 1.34kPa vacuum distillation, collect 83°C fraction, wash in 83% butyl oxalate solution 5 times, washed 6 times in 87% ethanedinitrile solution, and recrystallized in 93% 2-methylbutyric acid solution to obtain 322 g of crystalline 4-chlorobenzaldehyde with a yield of 92%.
Embodiment 3
[0021] The synthetic method of fenalu pharmaceutical intermediate 4-chlorobenzaldehyde comprises the steps:
[0022] Add 2.5mol of 2-methyl-3-bromo-5-chlorophenol and 3.6mol mass fraction of 87% 4-methyl-2-pentanone solution in the reaction vessel, control the stirring speed to 210rpm, and raise the solution temperature To 49°C, add 3.4mol molybdenum pentachloride powder in 6 times, continue to react for 50min after the addition, 1.36kPa vacuum distillation, collect the fraction at 84°C, wash in 86% butyl oxalate solution 5 times, washed 6 times in ethanedinitrile solution with a mass fraction of 91%, and recrystallized in a 2-methylbutyric acid solution with a mass fraction of 96%, to obtain 329 g of crystalline 4-chlorobenzaldehyde with a yield of 94%.
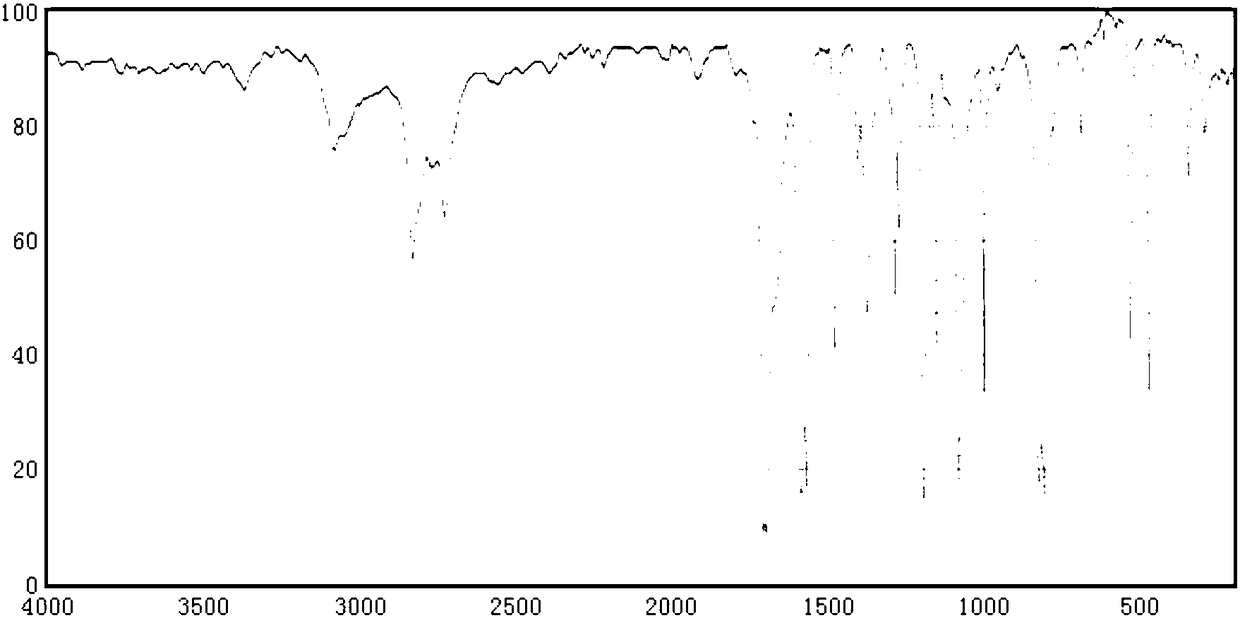
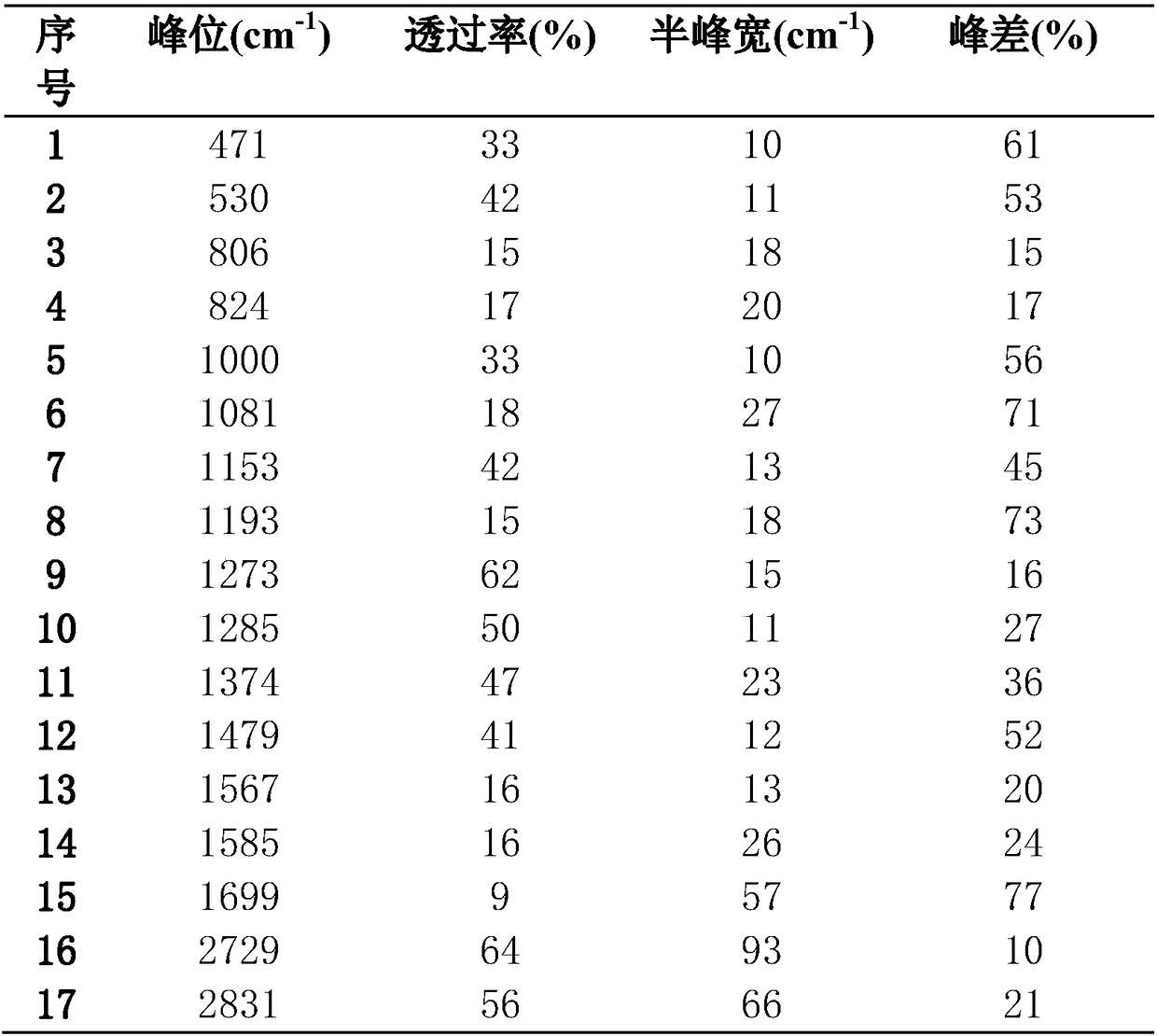
[0023] Finished product 4-chlorobenzaldehyde is done infrared analysis, and infrared spectrogram is as follows figure 1 Shown, Table 1 is the analysis data.
[0024] Table 1 Spectrum peak data table
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com