Biomimetic chitosan filling agent for preventing capsule contracture and preparation method thereof
A technology of capsular contracture and chitosan, applied in the fields of medical formula, tissue regeneration, medical science, etc., can solve problems such as increased incidence of capsular contracture, poor effect, increased incidence of capsular contracture, etc., to prevent occurrence Development, reduction of inflammation, good physical barrier effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
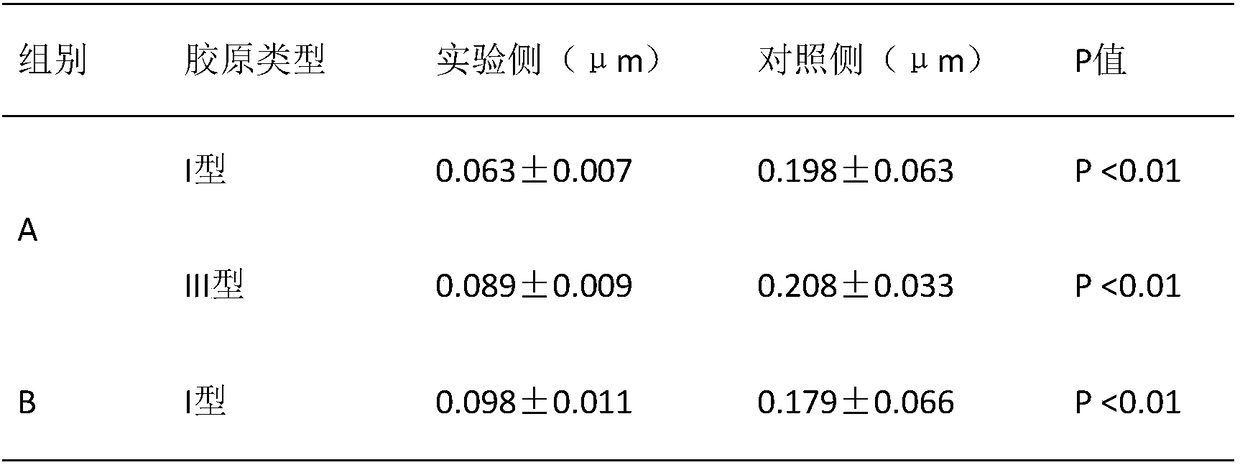
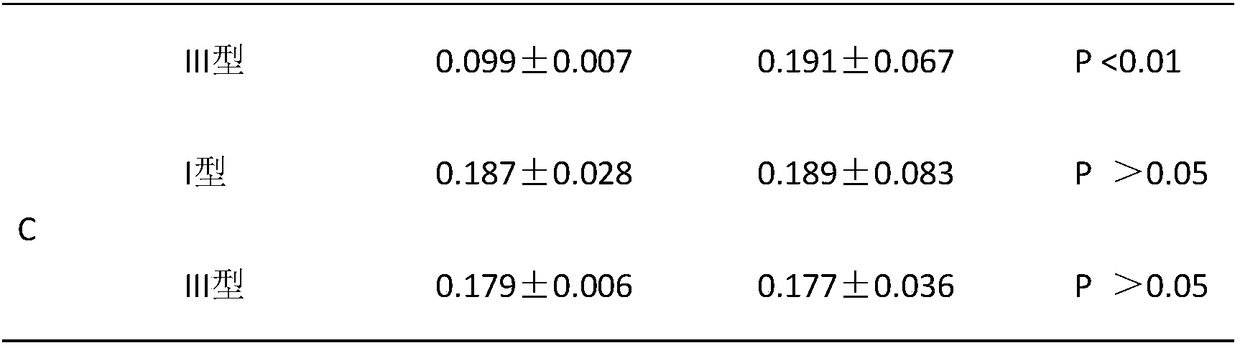
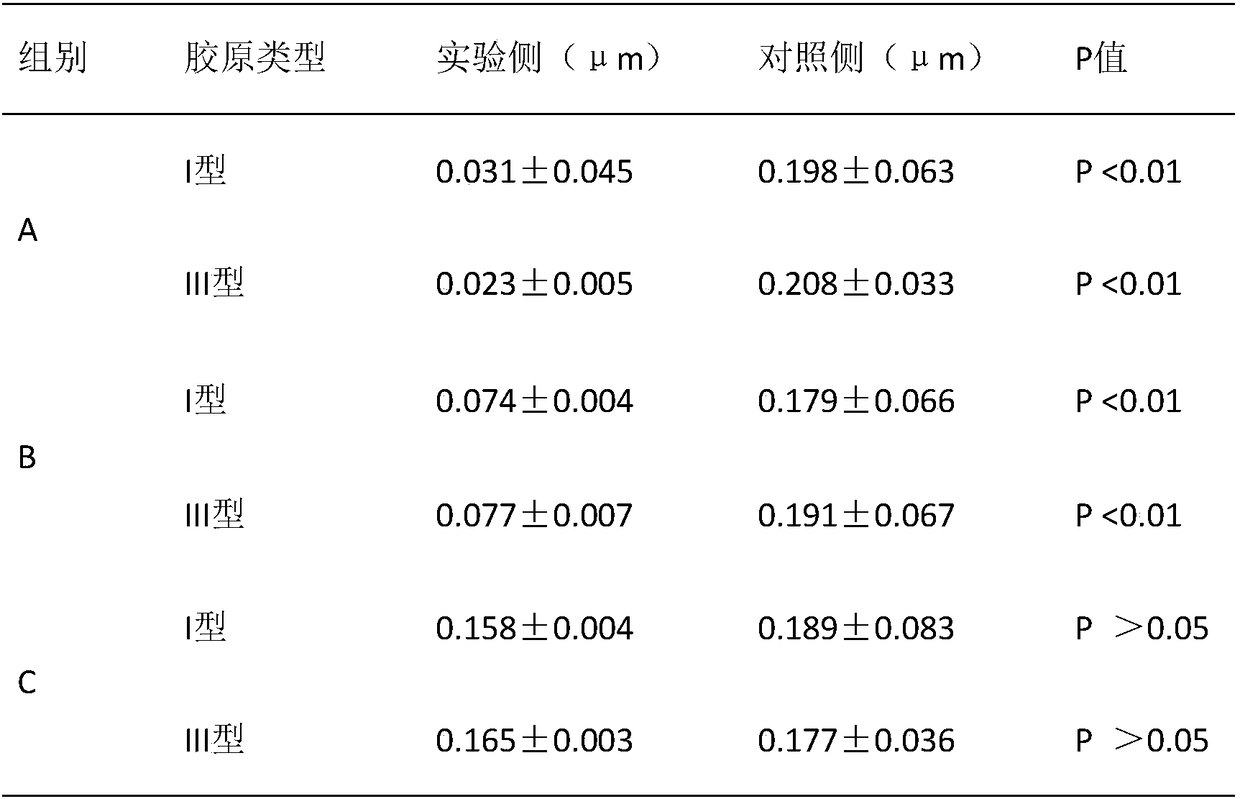
[0040] A biomimetic chitosan filler for preventing capsular contracture, comprising: 10wt‰ of hydroxybutyl chitosan gel, 10wt‰ of cross-linked sodium hyaluronate with a molecular weight of 2.5-6 million Daltons, 0.5 wt‰ polyethylene glycol, 0.02‰ sodium chloride, 0.03‰ potassium chloride, 1×10 10 Autologous adipose stem cells and phosphate buffer, the balance is water for injection, the amount of phosphate buffer added is to make the pH of the chitosan filler be 7.0, and finally prepared into 3mL chitosan filler, the chitosan filler The osmotic pressure of the agent is 300mOsmol / Kg.
[0041] The preparation method of above-mentioned chitosan filler is:
[0042] (1) Weigh the water for injection of 50wt% of the formula, and add the chitosan gel, cross-linked sodium hyaluronate, surfactant, isotonic regulator and autologous adipose stem cells into the container, according to the speed of 150rpm Stir evenly under the conditions of 5 hours and 20°C, add a pH regulator to adjust ...
Embodiment 2
[0045] A biomimetic chitosan filler for preventing capsular contracture, comprising: 0.005wt‰ of hydroxybutyl chitosan gel, 5wt‰ of cross-linked sodium hyaluronate with a molecular weight of 2.5-6 million Daltons, 100wt The mixture of ‰ butanediol and Tween 80, 50wt‰ of magnesium chloride, 1×10 5 Autologous adipose stem cells and boric acid, the balance is water for injection, the addition of boric acid is to make the pH of the chitosan filler be 6.5, and finally prepared into 3mL chitosan filler, the osmotic pressure of the chitosan filler is 270mOsmol / Kg.
[0046] The preparation method of above-mentioned chitosan filler is:
[0047] (1) Weigh the water for injection of 40wt% of the formula, and add the chitosan gel, cross-linked sodium hyaluronate, surfactant, isotonic regulator and autologous adipose stem cells into the container, according to the speed of 200rpm Stir evenly under the conditions of 6 hours and 25°C, add a pH regulator to adjust the pH to 6.5, and use a ...
Embodiment 3
[0050] A biomimetic chitosan filler for preventing capsular contracture, comprising: 5wt‰ of chitosan gel, 0.005wt‰ of cross-linked sodium hyaluronate with a molecular weight of 2.5-6 million Daltons, and 50wt‰ of Potassium chloride at temperature 80, 20wt‰, 1×10 10 Autologous adipose stem cells and hydrochloric acid, the balance is water for injection, the addition of hydrochloric acid is to make the pH of the chitosan filler be 7.5, and finally prepared into 3mL chitosan filler, the osmotic pressure of the chitosan filler is 320mOsmol / Kg.
[0051] The preparation method of above-mentioned chitosan filler is:
[0052] (1) Weigh the water for injection of 60wt% formula quantity, add chitosan gel, cross-linked sodium hyaluronate, surfactant, isotonic regulator and autologous adipose stem cells into the container together with the formula quantity, according to the speed of 100rpm Stir evenly under the conditions of 3 hours and 15°C, add a pH regulator to adjust the pH to 7.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com