Process for dehydrogenating alkanes to olefins
A technology for alkane dehydrogenation and olefin production, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of no significant improvement in overall performance, high-temperature instability of chromium species, high cost of precious metals, etc. , to achieve the effect of simple operation, inhibition of coking, and improvement of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also provides a preparation method of the catalyst, which is characterized in that the method comprises the following steps:
[0034] 1) Dry pretreatment of carbon material as template agent;
[0035] 2) Prepare a mixed aqueous solution of metal M salt and Zr salt;
[0036] 3) Add the mixed aqueous solution prepared in step 2) dropwise to the carbon material in step 1), and stir the resulting mixture to obtain a catalyst precursor;
[0037] 4) Drying and calcining the catalyst precursor in step 3) to obtain the catalyst MZr m O n .
[0038] In an embodiment of the present invention, the carbon material is selected from activated carbon or carbon black, such as granular activated carbon CGP, carbon black BP2000 or FW200; preferably carbon black, which has a specific surface area of 1100-1600 m 2 .g -1 , Preferably 1300-1500m 2 .g -1 The pore volume is 2-5mL / g, preferably 3-5mL / g. The carbon material can be prepared by a method known to those skilled in the...
Embodiment 1
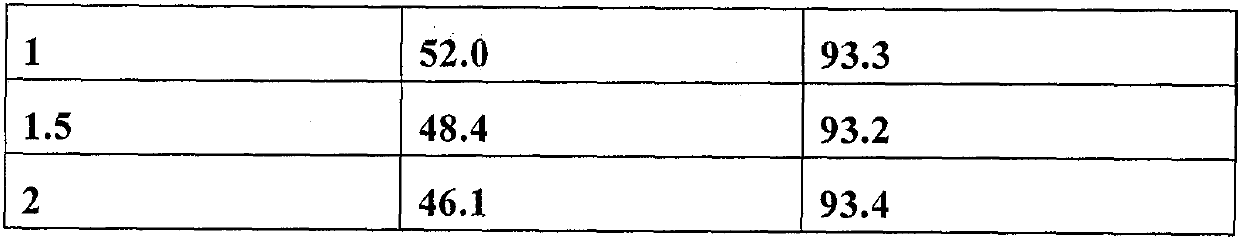
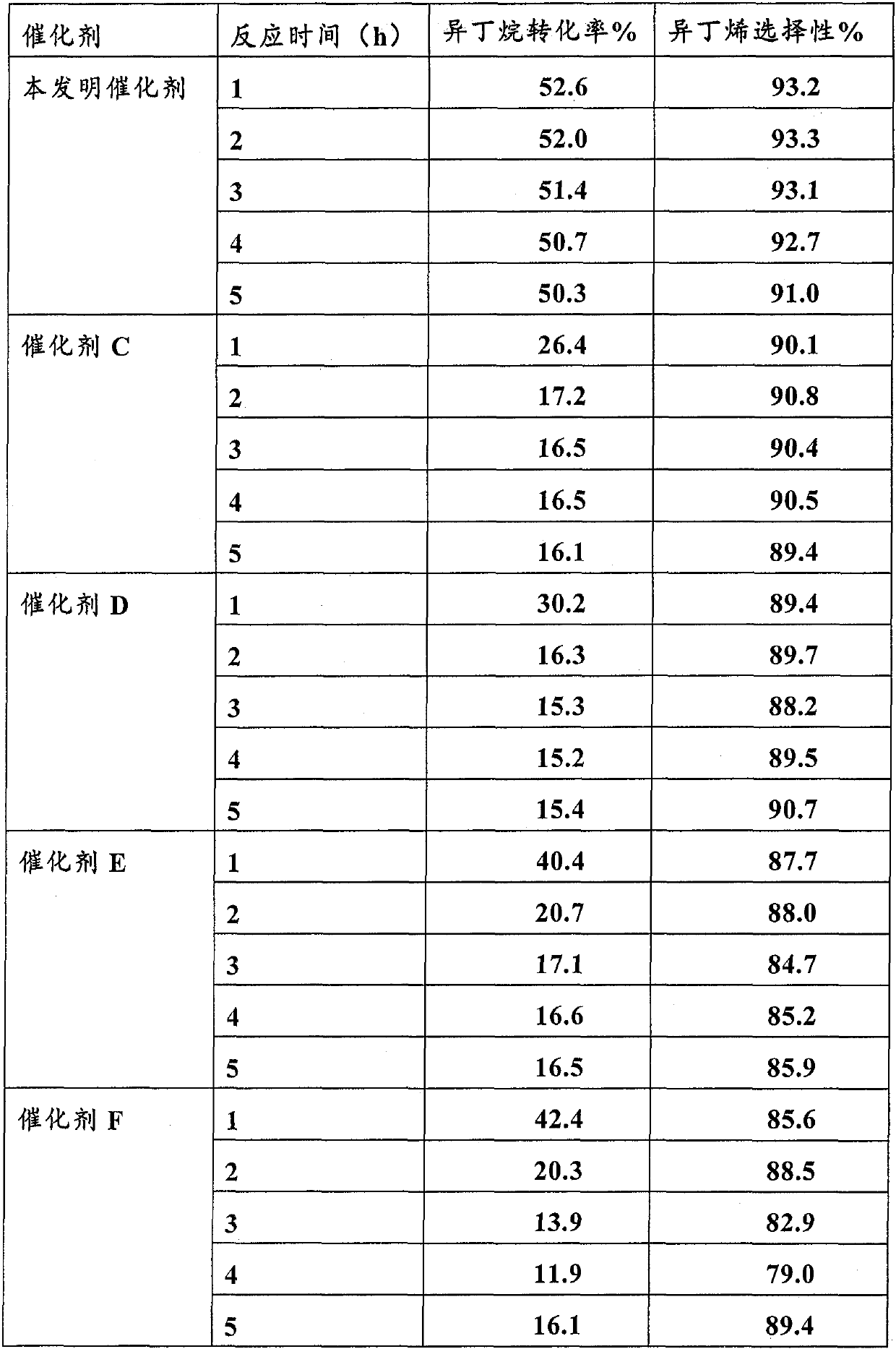
[0082] Put Cu(NO 3 ) 2 ·3H 2 O and ZrO (NO 3 ) 2 ·2H 2 O was prepared with deionized water to form a mixed aqueous solution of metal salt with a concentration of 0.15 mol / L, in which the molar ratio of Cu and Zr was 1:5. Then, it was sonicated at 40°C for 1 hour to obtain a clear solution. At room temperature 22°C, take 6g carbon material BP2000 and add 23.32g metal salt aqueous solution dropwise, stir for 1 hour, dry overnight at room temperature, dry at 80°C for 5 hours, and calcinate at 550°C for 20 hours. The obtained catalyst is denoted as CuZr 5 O n . The catalyst needs to be at 580℃, H 2 / N 2 Pre-reduction treatment under the atmosphere for 2 hours. See Table 1 for activity evaluation data.
Embodiment 2
[0084] Put Cu(NO 3 ) 2 ·3H 2 O and ZrO (NO 3 ) 2 ·2H 2 O is formulated with deionized water to form a mixed aqueous solution of metal salt with a concentration of 0.15 mol / L, in which the molar ratio of Cu and Zr is 1:10. Then, it was sonicated at 40°C for 1 hour to obtain a clear solution. At room temperature 22℃, take 6g carbon material BP2000 and add 23.44g metal salt aqueous solution dropwise, stir for 75 minutes, dry overnight at room temperature, dry at 80℃ for 5h, and calcinate at 550℃ for 20 hours. The obtained catalyst is denoted as CuZr 10 O n . The catalyst needs to be at 580℃, H 2 / N 2 Pre-reduction treatment under the atmosphere for 2 hours. See Table 1 for activity evaluation data.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com