1,1,1-triphenyl-N-(1-(pyridine-2-methylene) methylamine containing complex as well as preparation method and application thereof
A complex, triphenyl technology, applied in the field of chemical pharmacy, can solve the problems such as the anti-cancer activity needs to be improved, there is no semi-sandwich structure, etc., and achieve the effects of easy control of chemical components, low cost and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 50.0mg iridium dimer (formula (I) R1 is 1.2.3.4.5-pentamethylcyclopentadienyl, R2 is hydrogen, M is metallic iridium), 46.3mg 1,1,1-triphenyl -N-(1-(pyridine-2-methylene)methanamine was placed in a 250mL Schlenk bottle, vacuumed, and nitrogen gas was injected three times, and 20mL of analytically pure ethanol was added with a needle, and stirred at room temperature for 24h. Add 60 mg KPF6, spin dry with rotary evaporator, then dissolve with CH2Cl2, filter with diatomaceous earth, and recrystallize by diffusion method to obtain red crystals. Yield: 52.48mg (61.3%).
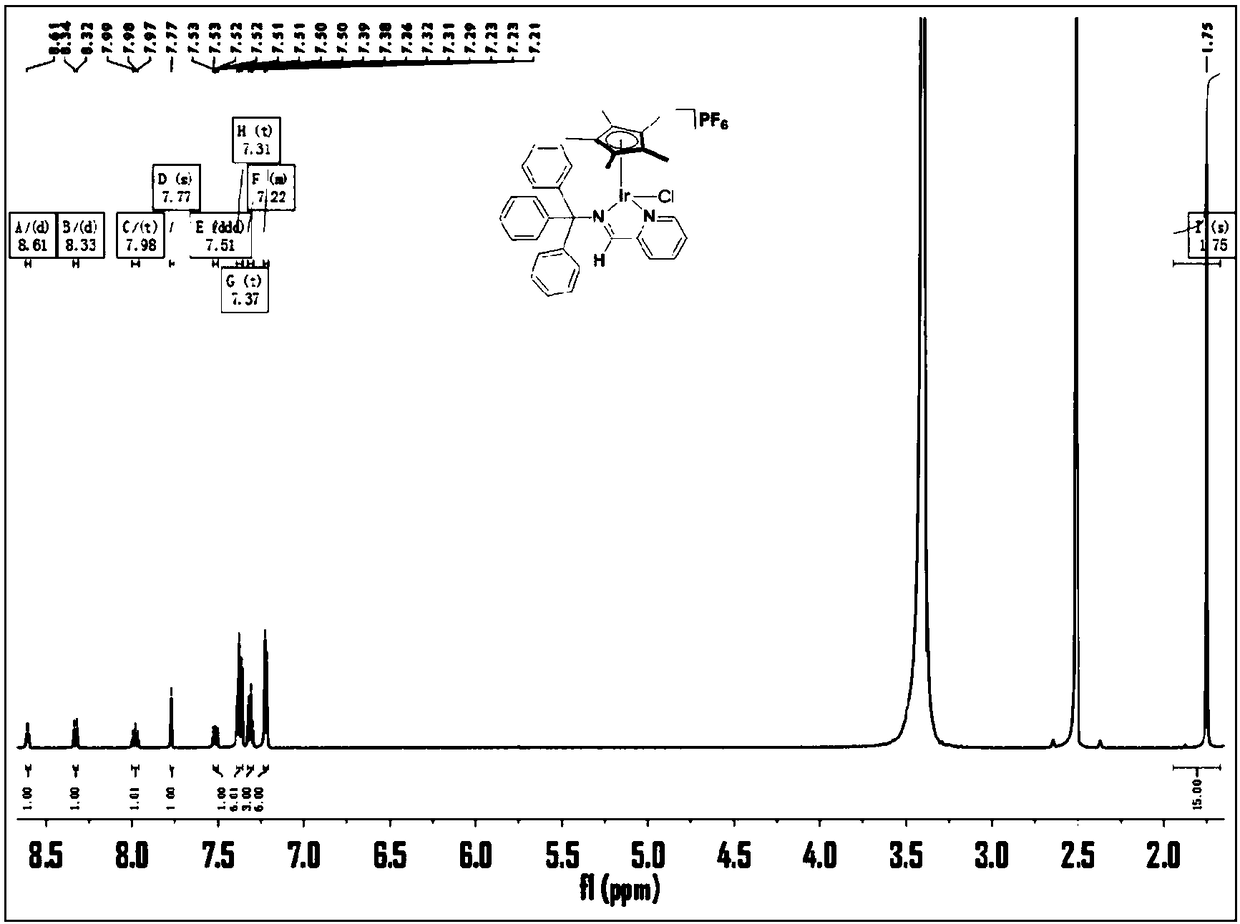
[0045] Such as figure 1 As shown, the NMR characterization is: [(η5-Cp*)Ir(N^N)Cl]PF6 (1). 1H NMR (500 MHz, DMSO) δ 8.61 (d, J = 4.7 Hz, 1H), 8.33 ( d, J = 7.9 Hz, 1H), 7.98 (t, J = 7.0Hz, 1H), 7.77 (s, 1H), 7.51 (ddd, J = 7.5, 4.8, 1.1 Hz, 1H), 7.37 (t, J = 7.5Hz, 6H), 7.31 (t, J = 7.3 Hz, 3H), 7.24 – 7.21 (m, 6H), 1.75 (s, 15H). Anal.Calcd. For [(η5- Cp*)Ir(N ^N)Cl]PF6 (856.3): C, 49.09; H, 4.12; N, 3.2...
Embodiment 2
[0047] 50.0mg iridium dimer (formula (I) R1 is 4-(2.3.4.5-tetramethylcyclopentadiene)-1'1-biphenyl, R2 is hydrogen, M is metallic iridium), 43.6 mg 1,1,1-Triphenyl-N-(1-(pyridine-2-yl)ethylidene)methanamine was placed in a 250mL Schlenk bottle, vacuumed, three times with nitrogen, and added with a needle 20 mL of analytically pure ethanol, stirred at room temperature for 24 hours, added 60 mg KPF6, spin-dried with a rotary evaporator, dissolved in CH2Cl2, filtered with diatomaceous earth, and recrystallized by diffusion method to obtain red crystals, the single crystal structure of complex 2 Such as figure 2 shown. Yield: 40.05 mg (40.3%).
[0048] Such as image 3 As shown, the NMR characterization is: [(η5-Cpxbiph )Ir(N^N)Cl]PF6 (2). Yield: 40.05mg, 40.3%.1H NMR (500 MHz, DMSO) δ 13.59 (d, J = 9.6 Hz, 2H), 9.54 (d, J =9.7 Hz, 3H), 8.80 (d, J = 5.4 Hz, 2H), 8.57 – 8.13 (m, 5H), 8.05 – 7.65 (m,8H), 7.64 – 7.23 (m, 8H), 1.83 (dd, J = 33.5, 10.8 Hz, 12H). Anal. Calcd. For[...
Embodiment 3
[0050] 50.0mg ruthenium dimer (formula (III) R1 is phenyl, R2 is hydrogen, M is metal ruthenium), 63.1mg 1,1,1-triphenyl-N-(1-(pyridine-2-yl )Ethylene)methylamine was placed in a 250mL Schlenk bottle, vacuumed, and nitrogen gas was injected three times, 20mL of analytically pure ethanol was added with a needle, stirred at room temperature for 24 hours, 60 mg of KPF6 was added, and spun with a rotary evaporator After drying, dissolve with CH2Cl2, filter with diatomaceous earth, and recrystallize by diffusion method to obtain red crystals. The single crystal structure of complex 3 is as follows: Figure 4 shown. Yield: 45.43 mg (61.3%).
[0051] Such as Figure 5 As shown, the NMR characterization is: [(η6-bz )Ru(N^N)Cl]PF6 (3). 1H NMR (500 MHz, DMSO) δ 9.63 (d, J = 5.3 Hz, 1H), 8.78 (s , 1H), 8.32 (d, J = 7.9 Hz, 1H), 8.26 (t, J = 8.2 Hz, 1H), 7.89 – 7.86 (m, 1H), 7.45 (ddd, J = 27.3, 19.4, 7.3Hz, 15H), 5.61 (s, 6H). Anal. Calcd. For [(η6- bz )Ir(N^N)Cl]PF6 (708.04): C,52.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com