Preparation method and intermediate of pinoxaden
A technology of pinoxaden and intermediates, applied in the field of organic synthesis, can solve the problems of high cost, three-waste pollution, halogen corrosion, etc., and achieves the effects of low cost, less three-waste and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
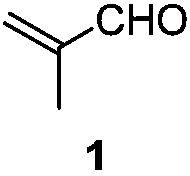
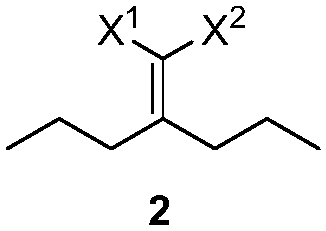
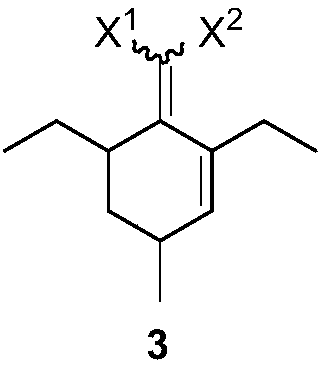
[0032] Example 1: Preparation of 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene)malononitrile
[0033] Add 32.4g (0.20mol) of 2-(4-heptyl)malononitrile, 14.0g (0.20mol) of 2-methacrolein and 2.2g (0.02mol) of triethylenediamine to toluene in sequence, and at 130 Reaction at ℃. After the reaction is complete, lower the temperature, wash with 1N dilute hydrochloric acid, extract with ethyl acetate, dry, concentrate and distill to obtain the product 2-(2,6-diethyl-4-methyl-2-en-1-cyclohexylene) propane Nitrile 39.4g, yield 92%. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.14-6.14(m,1H),3.08-3.04(m,1H),2.82-2.75(m,1H),2.57-2.46(m,2H),2.04-2.01(m, 1H), 1.56-1.51(m, 2H), 1.48-1.41(m, 1H), 1.12-1.01(m, 6H), 1.00-0.98(m, 3H). 13 C NMR (CDCl 3 ,125MHz): δ175.12, 148.74, 134.78, 113.99, 113.74, 43.75, 34.75, 28.13, 16.55, 15.52, 20.91, 13.59, 11.98.
Embodiment 2
[0034] Example 2: Preparation of methyl 2-cyano-2-(2,6-diethyl-4-methyl-1-cyclohexenylene)acetate
[0035] Add 58.6g (0.300mol) of 2-cyano-3-propyl-2-enhexanoic acid methyl ester, 27.3g (0.390mol) of 2-methacrolein and 30.3g (0.300mol) of triethylamine to toluene in sequence In, the temperature was raised to reflux until the reaction was complete. Wash with warm, 1N dilute hydrochloric acid, dry, and concentrate to obtain 62.8 g of methyl 2-cyano-2-(2,6-diethyl-4-methyl-1-cyclohexenylidene) acetate. The yield is 84%. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.02-5.90(m,1H),3.83-3.82(m,3H),3.63-3.07(m,1H),2.91-2.44(m,2H),2.22-1.95(m, 2H), 1.58-1.42(m, 3H), 1.08-1.04(m, 4H), 1.00-0.90(m, 5H).
Embodiment 3
[0036] Embodiment three: the preparation of 2-(2,6-diethyl-4-methylphenyl) malononitrile
[0037] Heat 10g (0.05mol) of 2-(2,6-diethyl-4-methyl-2-en-1-cyclohexylene)malononitrile and 0.5g of Pd / C to 220°C in a nitrogen atmosphere to react After the reaction is complete, the temperature is lowered, the catalyst is removed by filtration, and washed with a small amount of solvent. The organic phase was extracted with 1N aqueous sodium hydroxide solution, the aqueous phase was washed with methyl tert-butyl ether, and the pH was adjusted to 3-5 with concentrated hydrochloric acid. The aqueous phase was then extracted twice with ethyl acetate. The organic phase was dried and concentrated to obtain 8.4 g of the product 2-(2,6-diethyl-4-methylphenyl)malononitrile, with a yield of 85%. 1 H NMR (CDCl 3,500MHz,TMS):δ7.00(s,2H),5.29(s,1H),2.81(q,J=7.5Hz,4H),2.34(s,3H),1.32(t,J=7.5Hz, 6H). 13 C NMR (CDCl 3 ,125MHz): δ142.66, 140.73, 128.74, 120.00, 112.24, 26.48, 21.21, 21.13, 15.03....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com