A kind of method for preparing pinoxaden and its intermediate
A pinoxaden and intermediate technology, applied in the field of organic synthesis, can solve the problems of high cost, three wastes pollution, halogen corrosion, etc., and achieve the effect of low cost, less three wastes, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
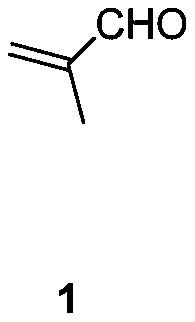
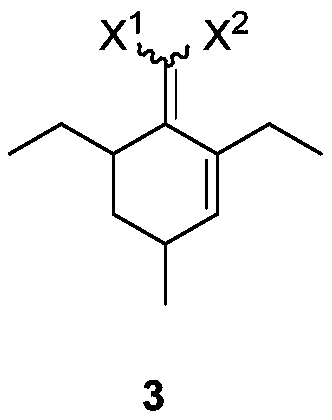
[0032] Example 1: Preparation of 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene) malononitrile
[0033] 32.4g (0.20mol) of 2-(4-heptidene)malononitrile, 14.0g (0.20mol) of 2-methacrolein and 2.2g (0.02mol) of triethylenediamine were successively added to toluene, at 130 reaction at ℃. After the reaction is complete, the temperature is lowered, washed with 1N dilute hydrochloric acid, extracted with ethyl acetate, dried, concentrated and distilled to obtain the product 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene) propanedi Nitrile 39.4g, yield 92%. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.14-6.14(m,1H),3.08-3.04(m,1H),2.82-2.75(m,1H),2.57-2.46(m,2H),2.04-2.01(m, 1H), 1.56-1.51 (m, 2H), 1.48-1.41 (m, 1H), 1.12-1.01 (m, 6H), 1.00-0.98 (m, 3H). 13 C NMR (CDCl 3 , 125MHz): δ175.12, 148.74, 134.78, 113.99, 113.74, 43.75, 34.75, 28.13, 16.55, 15.52, 20.91, 13.59, 11.98.
Embodiment 2
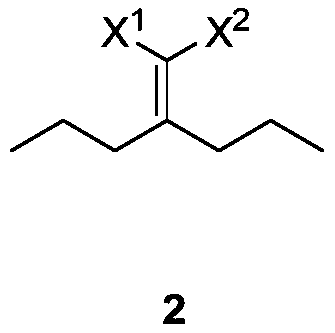
[0034] Example 2: Preparation of methyl 2-cyano-2-(2,6-diethyl-4-methyl-1-cyclohexenylene) acetate
[0035] 58.6g (0.300mol) of methyl 2-cyano-3-propyl-2-enehexanoate, 27.3g (0.390mol) of 2-methacrolein and 30.3g (0.300mol) of triethylamine were added to toluene in turn During the reaction, the temperature was raised and refluxed until the reaction was complete. The warm, 1N dilute hydrochloric acid was washed, dried, and concentrated to obtain 62.8 g of methyl 2-cyano-2-(2,6-diethyl-4-methyl-1-cyclohexenylidene)acetate in a yield of 62.8 g. 84%. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.02-5.90(m,1H),3.83-3.82(m,3H),3.63-3.07(m,1H),2.91-2.44(m,2H),2.22-1.95(m, 2H), 1.58-1.42 (m, 3H), 1.08-1.04 (m, 4H), 1.00-0.90 (m, 5H).
Embodiment 3
[0036] Example 3: Preparation of 2-(2,6-diethyl-4-methylphenyl)malononitrile
[0037] 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene) malononitrile 10g (0.05mol) and Pd / C 0.5g were heated to 220℃ in nitrogen atmosphere to react , after the reaction is complete, the temperature is lowered, the catalyst is removed by filtration, and washed with a small amount of solvent. The organic phase was extracted with 1N aqueous sodium hydroxide solution, the aqueous phase was washed with methyl tert-butyl ether, and the pH was adjusted to 3-5 with concentrated hydrochloric acid. The aqueous phase was then extracted twice with ethyl acetate. The organic phase was dried and concentrated to obtain 8.4 g of 2-(2,6-diethyl-4-methylphenyl)malononitrile with a yield of 85%. 1 H NMR (CDCl 3,500MHz,TMS):δ7.00(s,2H),5.29(s,1H),2.81(q,J=7.5Hz,4H),2.34(s,3H),1.32(t,J=7.5Hz, 6H). 13 C NMR (CDCl 3 , 125MHz): δ142.66, 140.73, 128.74, 120.00, 112.24, 26.48, 21.21, 21.13, 15.03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com