Expressing method and application of micromolecule thioesterase
A molecular thioester and expression method technology, applied in the biological field, can solve the problems of complicated operation steps, toxicity of chemical reagents, inability to meet market demand, etc., and achieve the effect of improving expression amount and solubility, and promoting correct folding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: PCR amplification of Escherichia coli acyl-CoA thioesterase gene ydiI
[0043] According to Escherichia coli acyl-CoA thioesterase gene ydiI design PCR amplification primers, the nucleotide sequence of the upstream primer is shown in SEQ ID NO.1, the nucleotide sequence of the downstream primer is shown in SEQ ID NO.2;
[0044] Wherein, the nucleotide sequence of Escherichia coli acyl-CoA thioesterase gene ydiI is shown in SEQ ID NO.3, and the amino acid sequence of the expressed small molecule thioesterase is shown in SEQ ID NO.4.
[0045] Using the above primers for PCR amplification, the reaction system is as follows:
[0046]
[0047] The PCR reaction conditions are as follows:
[0048]
[0049] PCR product recovery:
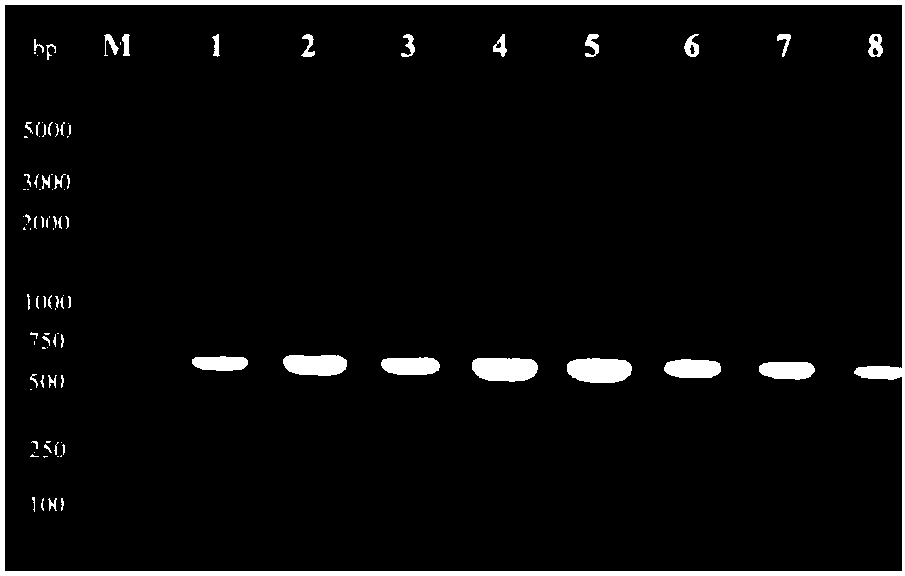
[0050] After PCR amplification, the length of the fragment was analyzed by 1% agarose gel electrophoresis, and the results were as follows: figure 1 As shown, the target band was excised according to the size of the fragment, an...
Embodiment 2
[0051] Embodiment 2: Construction of recombinant plasmid pET-28a-ydiI
[0052] The double enzyme digestion reaction of the PCR product recovered in Example 1 and the pET-28a plasmid vector containing the sumo tag, the reaction system is as follows:
[0053]
[0054] Reaction conditions: react at 37°C for 1.5h.
[0055] The PCR product and the plasmid vector were digested and purified by 1% agarose gel electrophoresis, and the target fragment was recovered using a DNA gel recovery kit.
[0056] Ligate the digested PCR product with the plasmid vector that has also been digested, and the ligation reaction system is as follows:
[0057]
[0058]
[0059] The above ligation reaction system was thoroughly mixed and then centrifuged for a few seconds, and the droplet on the tube wall was collected at the bottom of the tube, and ligated overnight at 16°C to obtain the recombinant plasmid pET-28a-ydiI.
Embodiment 3
[0060] Embodiment 3: Transformation of recombinant plasmid pET-28a-ydiI:
[0061] (1) Preparation of Competent Cells
[0062] ①Pick a single colony of Escherichia coli BL21 (DE3) (or pick a preserved strain) and inoculate it into 10ml of liquid LB medium, culture overnight at 37°C and 210rpm;
[0063] ② Inoculate 5ml of bacterial liquid into 500ml of LB medium, and culture at 37°C and 210rpm until the bacterial liquid is OD 600 is around 0.375;
[0064] ③ Place the bacterial solution on the ice-water mixture for 10 minutes, and pre-cool the 50ml centrifuge tube at the same time;
[0065] ④ Transfer the bacterial solution to a centrifuge tube, centrifuge at 3700 rpm for 10 minutes at 4°C to collect the bacterial cells;
[0066] ⑤Add 10mL pre-cooled 0.1M CaCl to each centrifuge tube 2 solution, resuspend the bacteria, and then add 30mL pre-cooled 0.1M CaCl 2 solution, mix it upside down, and let it stand on ice for 20 minutes;
[0067] ⑥Collect the bacteria by centrifugati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com