Synthesis technology of rebamipide
A synthesis process, the technology of rebamipide, which is applied in the field of synthesis technology of rebamipide, can solve the problems of low content of rebamipide, high production cost, difficult removal of impurities, etc., and achieves simple synthesis conditions and high yield. The effect of high and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
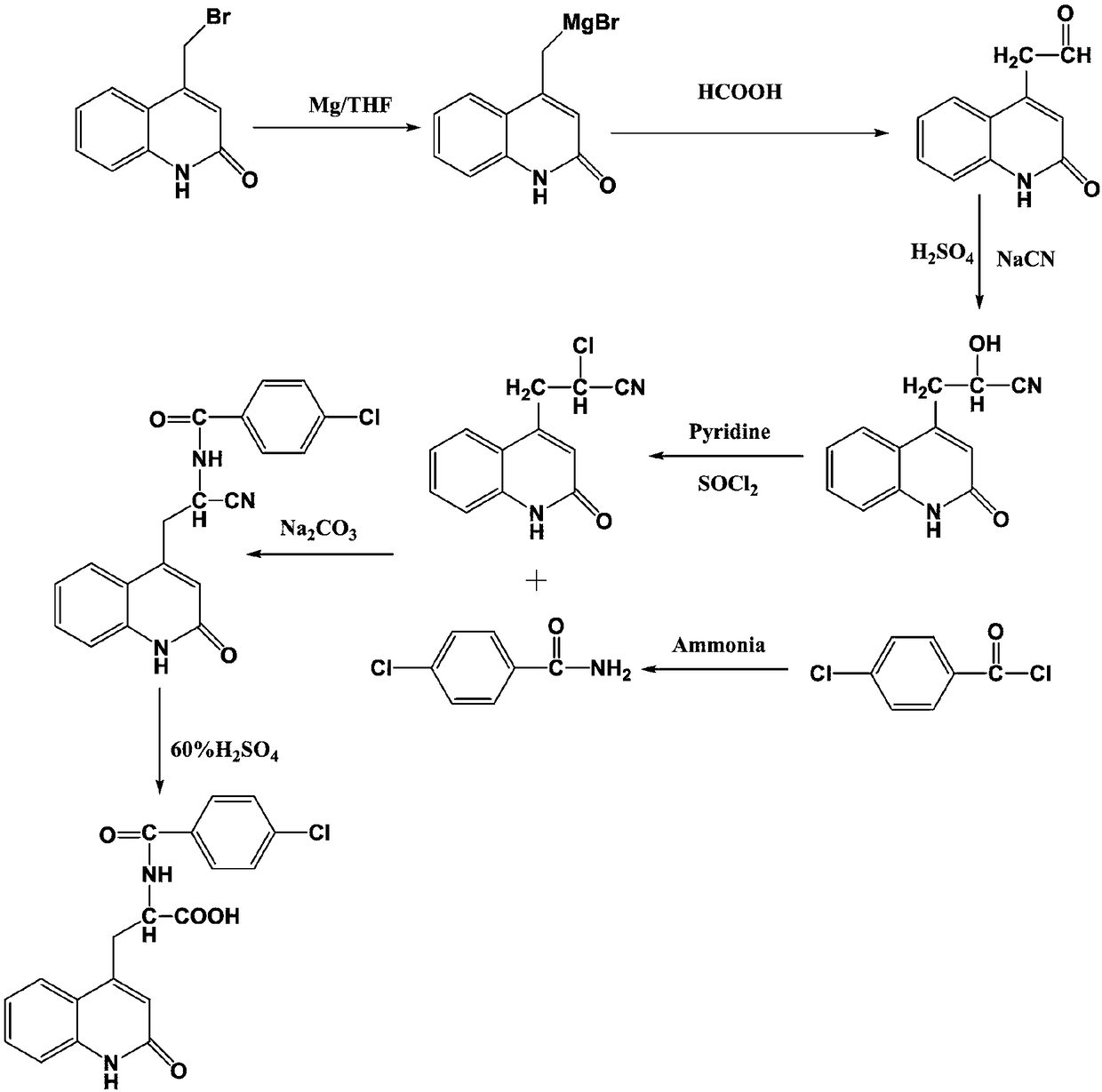
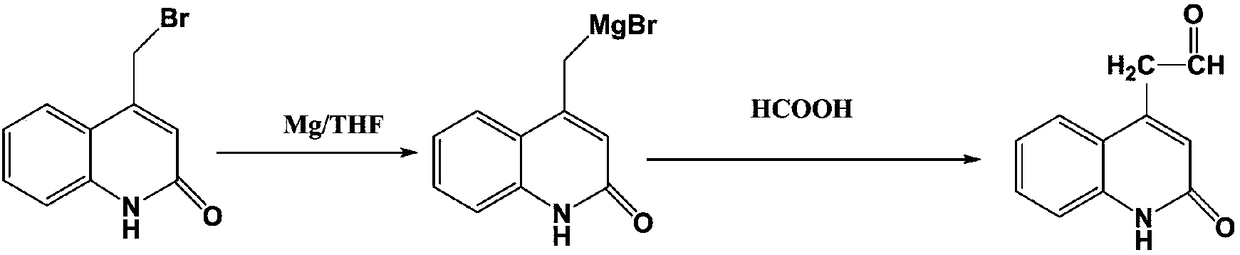
[0027] A kind of synthetic technique of the synthetic technique of rebamipide, such as figure 1 As shown, the specific synthesis process is as follows:
[0028] (1) Add 1L of concentrated ammonia water into the reaction vessel, then drop 1.843kg of 4-chlorobenzoyl chloride into it dropwise, control the reaction temperature within 30°C, and react by mechanical stirring for 4.5h to obtain 4-chlorobenzoyl chloride ;
[0029] (2) under the protection of nitrogen, add 1L anhydrous tetrahydrofuran and 72g magnesium chips in the container, and add a few drops of methyl iodide, be warming up to 80 ℃, add 695.3g4-bromomethylquinolone dropwise after stirring and dissolving, After the dropwise addition was completed, react at a constant temperature for 10 hours. After cooling down to room temperature, 101.26 g of formic acid was added dropwise. After the dropwise addition was complete, the temperature was raised to 70°C for 5 hours.
[0030] (3) 930g of product 1, 383.5g of sodium cyan...
Embodiment 2
[0034] A synthetic process of the synthetic process of rebamipide, the specific synthetic process is as follows:
[0035] (1) Add 1L of ammonia water into the reaction vessel, then drop 1.935kg of 4-chlorobenzoyl chloride into it dropwise, control the reaction temperature within 30°C, and react by mechanical stirring for 4.5h to obtain 4-chlorobenzoyl chloride;
[0036] (2) under the protection of nitrogen, add 1L anhydrous tetrahydrofuran and 72g magnesium chips in the container, and add a few drops of methyl iodide, be warming up to 80 ℃, add 704.9g4-bromomethylquinolone dropwise after stirring and dissolving, After the dropwise addition was completed, react at a constant temperature for 10 hours. After cooling down to room temperature, 115.07 g of formic acid was added dropwise. After the dropwise addition was complete, the temperature was raised to 70°C for 5 hours.
[0037] (3) 930g of product 1, 393.25g of sodium cyanate and 396.9g of sulfuric acid solution were added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com