A kind of pemetrexed disodium freeze-dried powder for injection
A technology for pemetrexed disodium and freeze-dried powder injection, which is applied in the field of pemetrexed disodium freeze-dried powder for injection, can solve the problems of complicated operation, rough freeze-drying process, poor stability of main drug and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
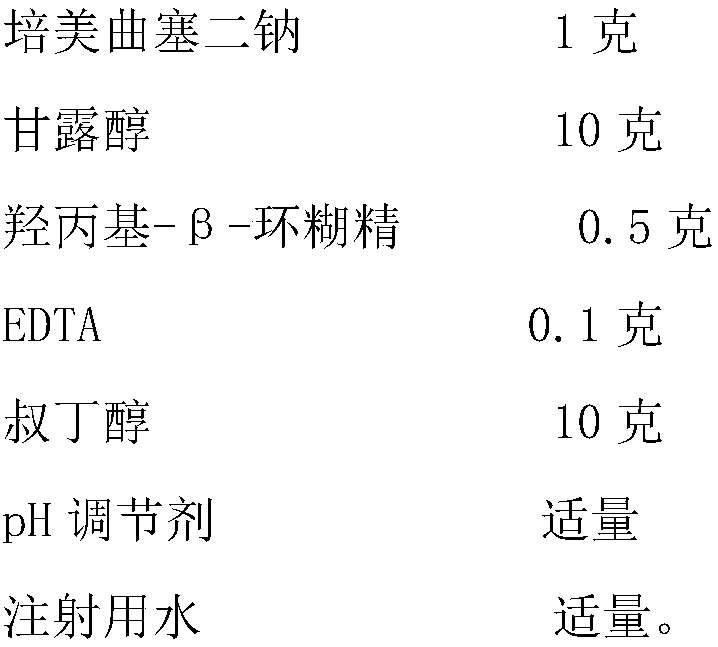
[0039] Embodiment 1: a kind of pemetrexed disodium freeze-dried powder injection, by weight ratio, its formula composition is as follows:
[0040]
[0041] The preparation process is:
[0042] (1) Take the hydroxypropyl-beta-cyclodextrin of the prescribed amount, add water for injection, stir and dissolve, then add the tert-butanol and EDTA of the prescribed amount, stir and dissolve, and set aside;
[0043] (2) Add mannitol and pemetrexed disodium of recipe quantity in step (1), stir to make main ingredient dissolve completely;
[0044] (3) Add the gac of recipe quantity in step (2), stir 15 minutes, decarbonize;
[0045] (4) Add water for injection to the full amount, and use L-arginine to adjust the pH value to 8.0;
[0046] (5) Filling: filling the medicinal solution in ampoules and freeze-drying;
[0047] (6) Freeze-drying curve: After filling the product into the box, cool it down to -30±5°C for pre-freezing and keep it for 1-3 hours; heat it up to -10±5°C, keep it...
Embodiment 2
[0049] Embodiment 2: a kind of pemetrexed disodium freeze-dried powder injection, by weight ratio, its formula composition is as follows:
[0050]
[0051]
[0052] The preparation process is the same as in Example 1.
Embodiment 3
[0053] Embodiment 3: a kind of pemetrexed disodium freeze-dried powder injection, by weight ratio, its formula composition is as follows:
[0054]
[0055] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com