Diacylhydrazine derivatives as well as preparation method and application thereof
A derivative, bishydrazide technology, applied in the field of synthesis of agricultural chemical insecticides, to achieve the effect of improving fat solubility and high insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
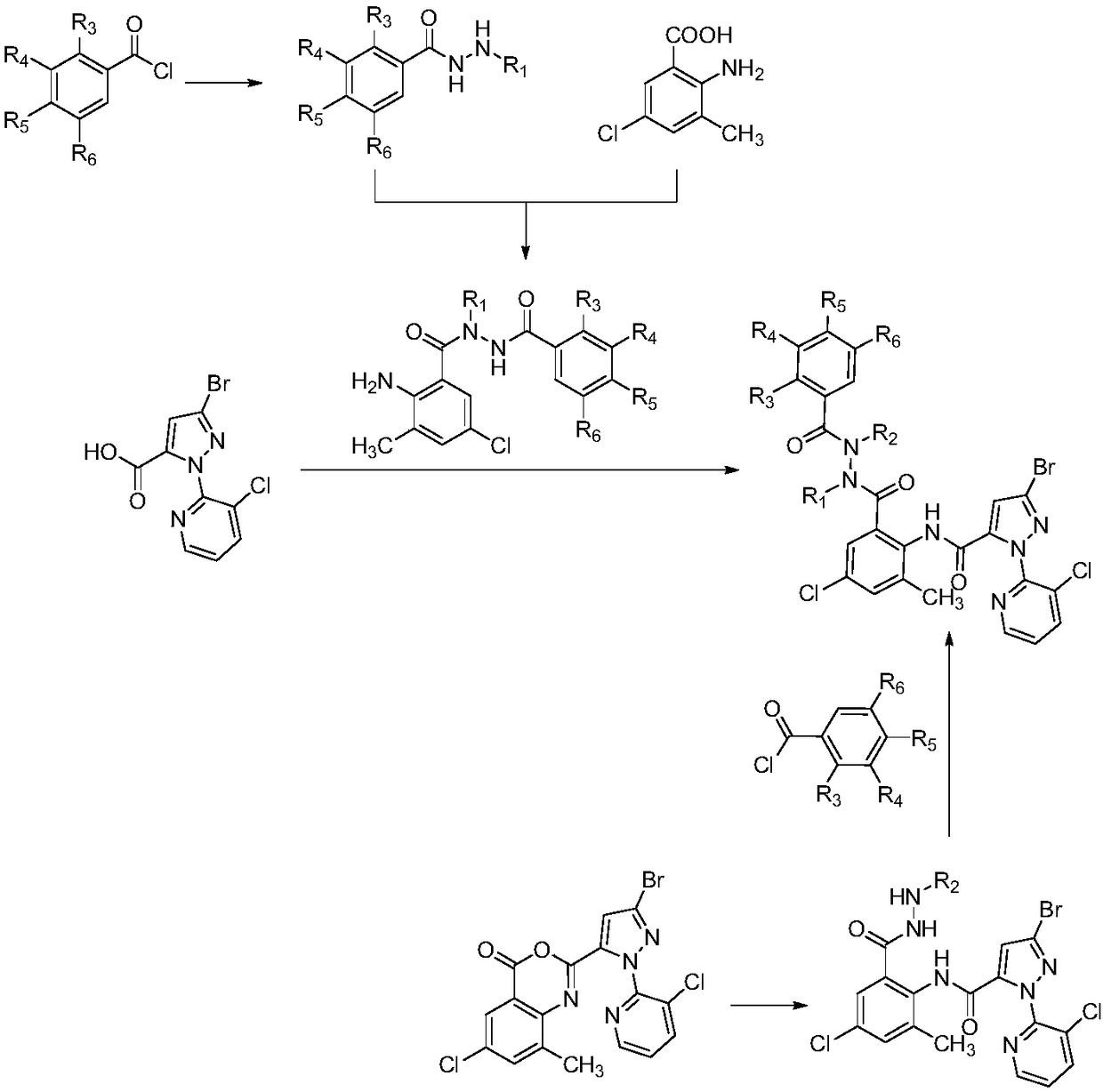
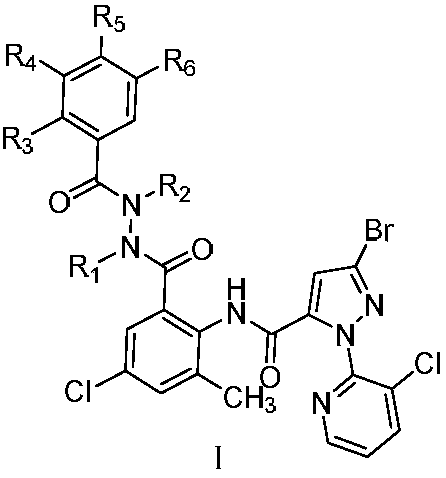
[0058] 3-Bromo-N-(2-(1-tert-butyl-2-(2-chlorobenzoyl)-1-formylhydrazino)-4-chloro-6-methylphenyl)-1-( Synthesis of 3-chloro-2-pyridyl)-1H-pyrazole-5-carboxamide (derivative 01):
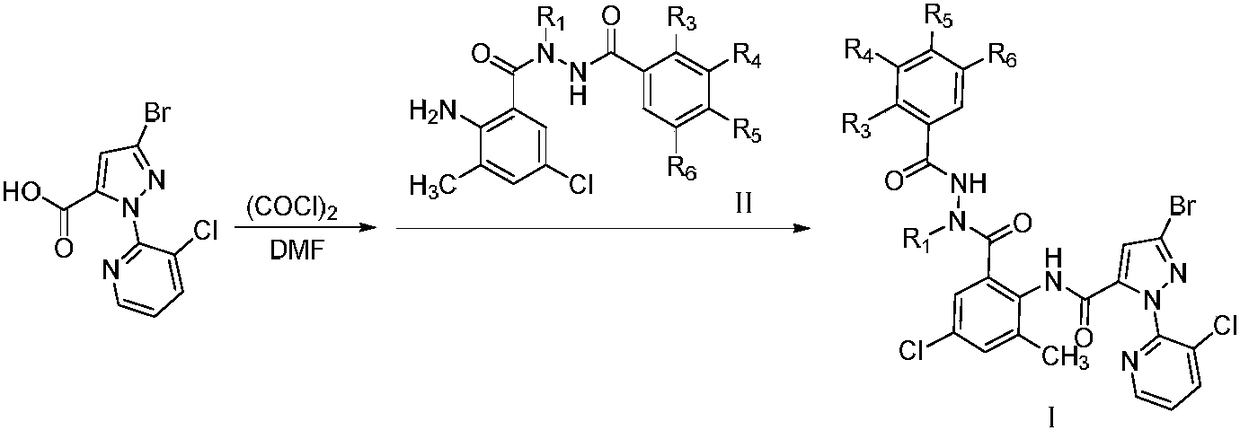
[0059] Step A: Preparation of N'-tert-butyl-2-chlorobenzohydrazide
[0060] At 0°C, add tert-butylhydrazine hydrochloride (20mmol) and sodium hydroxide to a 100mL round-bottomed flask, then add a mixed solvent of tetrahydrofuran / water, and slowly drop into a dichloromethane solution of 2-chlorobenzoyl chloride After completion of the dropwise reaction at room temperature, ethyl acetate was added after the reaction, and the organic layer was separated. After precipitation of the organic layer, the title compound was obtained, 4.2g of white powder, m.p.113-114°C.
[0061] Step B: Preparation of 2-amino-N-tert-butyl-5-chloro-N'-(2-chlorobenzoyl)-3-methylbenzohydrazide
[0062] In a 100 mL round bottom flask was added 2-amino-5-chloro-3-methylbenzoic acid (5 mmol), SOCl 2 , reflux, decompression to re...
Embodiment 2
[0066] 3-Bromo-N-(2-(1-tert-butyl-2-(3,5-dimethylbenzoyl)-1-carbohydrazino)-4-chloro-6-methylphenyl) - Synthesis of 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxamide (derivative 23):
[0067] Step A: Preparation of N'-tert-butyl-3,5-dimethylbenzohydrazide
[0068] At 0°C, add tert-butylhydrazine hydrochloride (20mmol) and sodium hydroxide to a 100mL round-bottomed flask, then add a mixed solvent of tetrahydrofuran / water, and slowly drop in 3,5-dimethylbenzoyl chloride Dichloromethane solution, dropwise, react at room temperature, add ethyl acetate after the reaction, separate the organic layer, and precipitate the organic layer to obtain the title compound, 4.1g white powder, m.p.117-118°C.
[0069] Step B: Preparation of 2-amino-N-tert-butyl-5-chloro-N'-(3,5-dimethylbenzoyl)-3-methylbenzohydrazide
[0070] In a 100 mL round bottom flask was added 2-amino-5-chloro-3-methylbenzoic acid (5 mmol), SOCl 2 , reflux, decompression to remove excess SOCl 2 , to obtain the crude aci...
Embodiment 3
[0074] 3-Bromo-N-(2-(2-tert-butyl-2-(3,5-dimethylbenzoyl)-1-carbohydrazino)-4-chloro-6-methylphenyl) - Synthesis of 1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxamide (derivative 34):
[0075] Step A: Preparation of 3-bromo-N-(2-(2-tert-butyl-1-carbohydrazino)-4-chloro-6-methylphenyl)-1-(3-chloro-2-pyridine base)-1H-pyrazole-5-carboxamide
[0076] Compound 6-chloro-2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4H-3,1-benzox Dissolve oxazin-4-one (5mmol) and tert-butylhydrazine hydrochloride (10mmol) in dimethyl sulfoxide, slowly drop into 1,8-diaza-bicyclo(5,4,0)undeca- 7-ene, dropwise, reacted at room temperature, added ethyl acetate after the reaction, separated the organic layer, and precipitated the organic layer to obtain the title compound, 1.6g white powder, m.p.211-212°C.
[0077] Step B: Preparation of 3-bromo-N-(2-(2-tert-butyl-2-(3,5-dimethylbenzoyl)-1-carbohydrazino)-4-chloro-6-methanol phenyl)-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxamide (der...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com