Preparation methods of lithium nickel cobalt aluminum oxide and composite material thereof
A technology of nickel cobalt lithium aluminate and composite materials, which is applied to active material electrodes, electrical components, electrochemical generators, etc., can solve problems such as easy water absorption, and achieve the effect of simple operation in the preparation process and easy large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
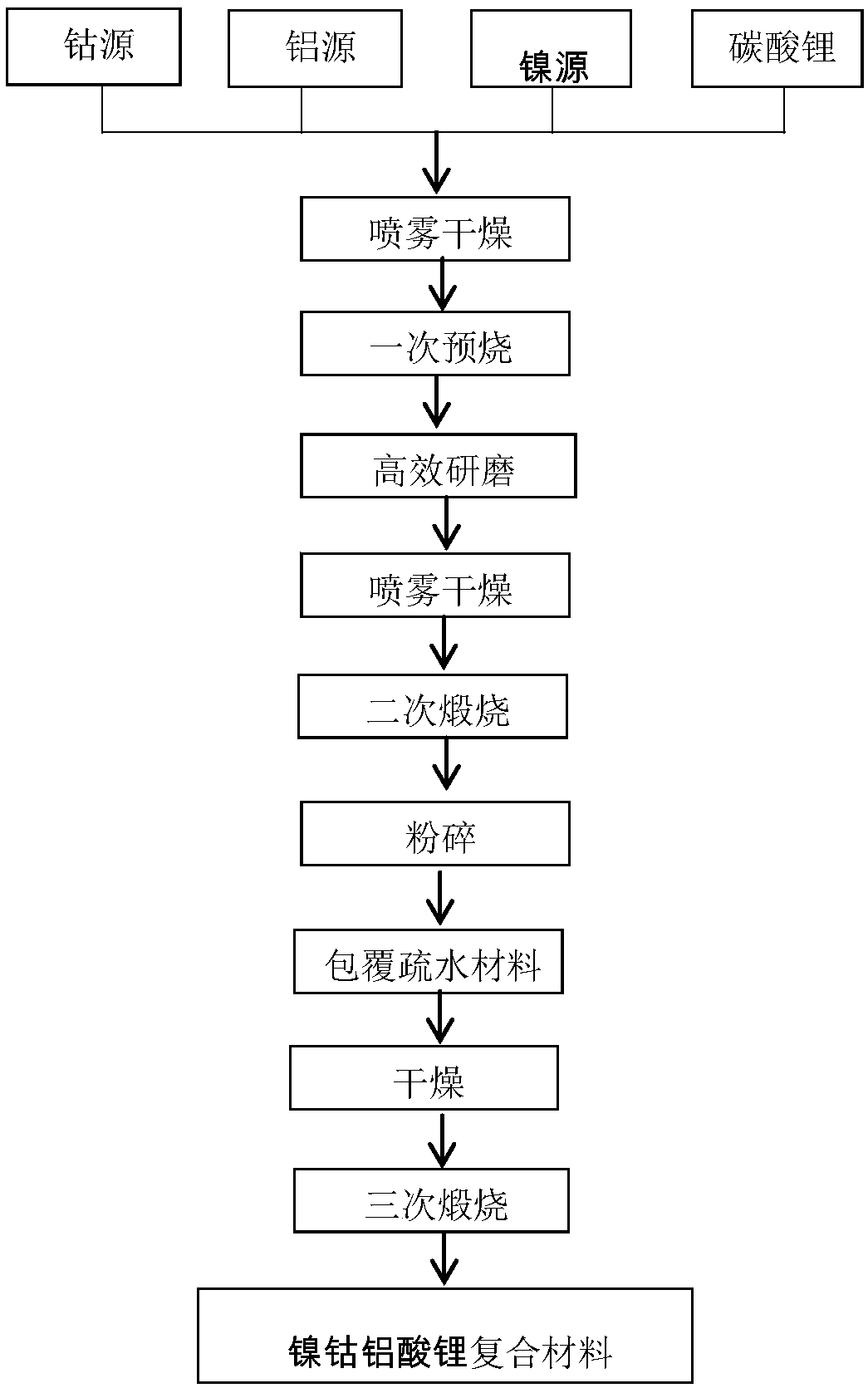
[0032] Lithium nickel cobalt aluminate LiNi of an embodiment 1-x-y co x al y m z o 2+z A method for preparing a composite material, comprising the steps of:
[0033] (1) dissolving the soluble nickel salt and the soluble cobalt salt in the first solvent and mixing to obtain the first mixed solution;
[0034] Preferably, in the first mixed solution, the molar ratio of nickel to cobalt is 6˜9:1. Soluble nickel salts include nickel nitrate and nickel chloride. Soluble cobalt salts include cobalt nitrate and cobalt chloride. The solvent in the first mixed solution is deionized water. The total concentration of solute in the first mixed solution is preferably 1mol / L;
[0035] (2) Prepare lithium compound solution, soluble aluminum salt solution and soluble metal M salt solution respectively, wherein metal M is selected from at least one of Mg, Be, Ca, Sr, Ba, Si, Zn, Y, Ga and In ; The order of preparing the above-mentioned first mixed solution and lithium compound solutio...
Embodiment 1
[0050] (1) 130.02g of Ni(NO3)2.6H2O and 15.385g of Co(NO3)2.6H2O were dissolved in deionized water to form a homogeneous 1mol / L first mixed solution.
[0051] (2) Dissolving 15.385 g of Co(NO3)2.6H2O in deionized water to form a homogeneous 1 mol / L solution.
[0052] (3) 5.92g of Al(NO3)3.9H2O was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0053] (4), 2.695g of Mg(NO3)2 was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0054] (5), 37.89g of LiNO3 was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0055] (6), according to the chemical formula is LiNi0.85Co0.1Al0.05Mg0.03O2.03, the solution obtained in (2), (3), (4) and (5) is mixed uniformly to obtain the second mixed solution, then ( The first mixed solution obtained in 1) is slowly added dropwise to the second mixed solution, and the dropping time is 60 minutes. After obtaining a homogeneous solution, it is spray-dried to obtain a powdery uniformly dr...
Embodiment 2
[0061] (1) 130.02g of Ni(NO3)2.6H2O and 15.385g of Co(NO3)2.6H2O were dissolved in deionized water to form a homogeneous 1mol / L first mixed solution.
[0062] (2) Dissolving 15.385 g of Co(NO3)2.6H2O in deionized water to form a homogeneous 1 mol / L solution.
[0063] (3) 5.92g of Al(NO3)3.9H2O was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0064] (4), 2.695g of Mg(NO3)2 was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0065] (5), 37.89g of LiNO3 was dissolved in deionized water to form a homogeneous 1mol / L solution.
[0066] (6), according to the chemical formula is LiNi0.85Co0.08Al0.07Mg0.01O2.01, the solution obtained in (2), (3), (4) and (5) is mixed uniformly to obtain the second mixed solution, then ( The first mixed solution obtained in 1) is slowly added dropwise to the second mixed solution, and the dropping time is 90 minutes. After obtaining a homogeneous solution, spray dry to obtain a powdery uniformly dried mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com