Preparation method and applications of amorphous form iron oxyhydroxides (FeOOH) super particles
An iron oxyhydroxide, amorphous technology, applied in the preparation of amino hydroxyl compounds, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of complex catalyst preparation steps, easy poisoning of catalysts, high price, etc., and achieve excellent catalytic activity and selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of the amorphous iron oxyhydroxide super particle that the present invention proposes, comprises the following steps:
[0018] (1) The surfactant is added to ethylene glycol, the mass fraction of the surfactant is 1%-5%. After the surfactant is completely dissolved, ferric chloride hexahydrate is added in the reaction system, and the hexahydrate chloride The mass fraction of iron is 0.5%-1%, and the solution becomes blood red, which is recorded as the first solution; urea is added in the first solution, and the mass fraction percentage added is: urea: first solution=(0.5%-3% ): 1, to obtain the second solution; utilize an ultrasonic cleaning machine with a power of 120W h to carry out ultrasonic treatment on the second solution for 15-60min, and finally heat it in an oil bath at 80-200°C for 5-45min, and the reaction solution Change from yellow to yellow-green, which is recorded as the third solution; after the third solution is reflux heated for ...
Embodiment 1
[0025] Embodiment 1: Preparation of amorphous iron oxyhydroxide (α-FeOOH) superparticles
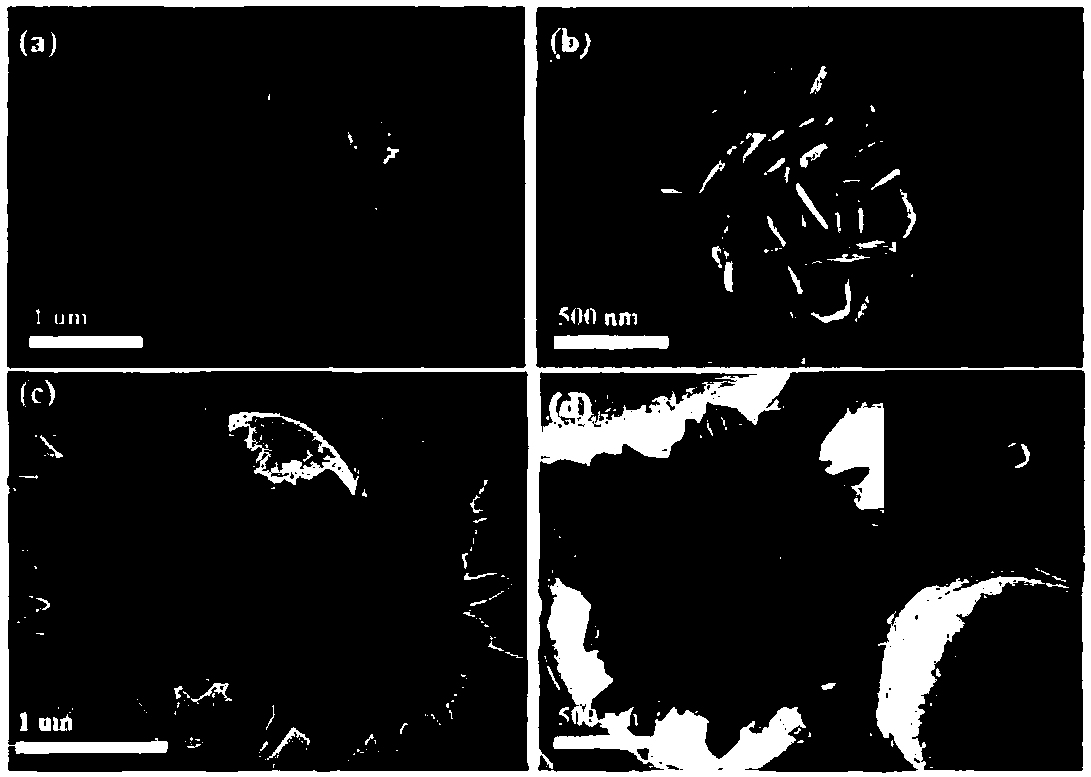
[0026] 1) First add 10 g of polyvinylpyrrolidone (PVP) to 400 mL of ethylene glycol (EG) in a 1000 mL round bottom flask. When the surfactant is completely dissolved, add 3.4g FeCl 3 ·6H 2 O, the solution turns blood red. After the solution was fully dispersed, 7.4 g of urea was added, and then the solution was ultrasonically treated for 30 min. The resulting mixture was transferred to an oil bath and heated to 135°C. After about 30 min, the reaction solution turned yellow, and then the reaction solution turned yellow-green, which indicated the formation of iron glycolate nanostructures. After refluxing for 45 min, the mixed solution turned green completely. Such as figure 2 As shown, 1 mL of solutions of different colors at different times were sampled, and the scanning electron microscope was used to characterize each sample. Such as figure 2 As shown in (a-b), when the solut...
Embodiment 2
[0028] Embodiment 2: Preparation of amorphous iron oxyhydroxide (α-FeOOH) superparticles
[0029] 1) In a 1000 mL round bottom flask, first add 14.2 g of tetrabutylammonium bromide (TBAB) into 400 mL of ethylene glycol (EG). When the surfactant is completely dissolved, add 2.4g FeCl 3 ·6H 2 O, the solution turns blood red. After the solution was fully dispersed, 5.4 g of urea was added, and then the solution was ultrasonically treated for 30 min. The resulting mixture was transferred to an oil bath and heated to 195°C. After about 15 min, a yellow precipitate started to appear, and then the mixture turned yellow-green, which indicated the formation of iron glycolate nanostructures. After refluxing for 45 min, the mixed solution turned green completely, then the reaction was stopped and the mixed solution was cooled to room temperature. The green precipitate was collected by filtration under reduced pressure and washed with 1500 mL of ethanol. The obtained filter cake was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com